![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
11 Cards in this Set
- Front
- Back
|
Regarding stereochemistry of Sn2 reactions: inversion of configuration because the _____ of the nucleophile reacts with the ____of the Electrophilic carbon |
HOMO-nucleophile LUMO-electrophillic carbon |
|
|
Reaction rate is a function of the _______ of reactants |
Concentration |
|
|
Strong nucleophiles |

|
|
|
Weak nucleophiles |
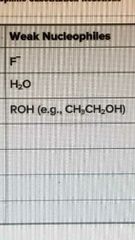
|
|
|
Lack of hindrance and a relatively weak base promotes |
Sn2 substitutions |
|
|
Substrate Crowding around the leaving group requires what mechanism |
Sn1 substitution |
|
|
In the presence of Lewis bases Alkyl halides primarily undergo... |
E2 eliminations |
|
|
Can have different carbon skeleton from their substrate because of 1,2 shift |
E1 eliminations |
|
|
For an unsymmetrical alkene treated with Br2 in H2O which C of the double bond is Br attracted to? |
The less substituted C |
|
|
For an unsymmetrical alkene treated with Br2 in H2O which C will OH be attracted to? |
The more substituted C on the double bond |
|
|
A strong base is generally a _______nucleophile. (strong or weak) |
Strong |

