![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
88 Cards in this Set
- Front
- Back
|
number of covalent bonds for H
|
1
|
|
|
# covalent bonds for C
|
4
|
|
|
# covalent bonds for N
|
3 (or 4)
|
|
|
# covalent bonds for 0
|
2
|
|
|
# covalent bonds for F
|
1
|
|
|
# covalent bonds for Cl
|
1
|
|
|
Number of covalent bonds for Br
|
1
|
|
|
# covalent bonds for I
|
1
|
|
|
isomers are
|
different compounds that have same molecular formula
|
|
|
Methane has what shape
|
tetrahedral
|
|
|
covelent bond
|
atoms share electrons
|
|
|
ionic bond
|
transfer of electrons
|
|
|
electroneg of Li
|
1.0
|
|
|
electro neg of Na
|
.9
|
|
|
electroneg of K
|
.8
|
|
|
# covalent bonds for Cl
|
1
|
|
|
Number of covalent bonds for Br
|
1
|
|
|
# covalent bonds for I
|
1
|
|
|
isomers are
|
different compounds that have same molecular formula
|
|
|
Methane has what shape
|
tetrahedral
|
|
|
covelent bond
|
atoms share electrons
|
|
|
ionic bond
|
transfer of electrons
|
|
|
electroneg of Li
|
1.0
|
|
|
electro neg of Na
|
.9
|
|
|
electroneg of K
|
.8
|
|
|
electroneg of K .
|
.8
|
|
|
electroneg of Rb
|
.8
|
|
|
electroneg of Cs
|
.7
|
|
|
electroneg of H
|
2.1
|
|
|
electroneg of B
|
2.0
|
|
|
electroneg of C
|
2.5
|
|
|
electroneg of N
|
3.0
|
|
|
electroneg of O
|
3.5
|
|
|
electroneg of F
|
4.0
|
|
|
electroneg of Si
|
1.8
|
|
|
electroneg of P
|
2.1
|
|
|
electroneg of S
|
2.5
|
|
|
electroneg of Cl
|
3.0
|
|
|
electroneg of Br
|
2.8
|
|
|
electro neg of I
|
2.5
|
|
|
formal charges formula
|
number of valence electrons - 1/2 the number of shared electrons (or the number of bonded electrons) minus the number of unshared electrons
|
|
|
how to determine major from minor resonance structures
|
1( obey octet rule, 2) cahrge separation 3) electronegativity has the correct charge
|
|
|
pi bonds join how
|
overlap on the side
|
|
|
sigma bonds bond how
|
end to end
|
|
|
Cis isomerism means what is on each side
|
the same
|
|
|
trans isomerism means what is on each side
|
opposites, (same across diagionally
|
|
|
amonia is what shape
|
trigonal pyramidal (
|
|
|
bond angle of tigonal pyramidal
|
107 degrees
|
|
|
what is an alkANE
|
hydrocarbons that do not have multipule bonds between carbon atoms
|
|
|
what is an alkENE
|
contains at lease one carbon carbon double bond
|
|
|
wht is an alkYNE
|
containc at least one carbon carbon triple bond
|
|
|
what is an aromatic compound
|
special type of ring. most common is the benzene ring
|
|
|
primary source of alkANES:
|
natural gas and petroleum, smaller alkanes are methane thru butane
|
|
|
what are the two simples alKENES
|
ethene and propene
|
|
|
what is the simplest alKYNE
|
ethyne (acetylene)
|
|
|
benzene ring looks like what
|
it is aromatic extra stable
|
|
|
which has dipole moments cis or trans?
|
cis- the pull is on the same side. on trans it pulls agains each other no movement
|
|
|
draw the phenyl group
|
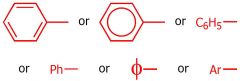
|
|
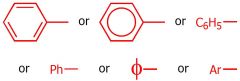
what group is this
|
Phenyl group
|
|
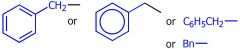
what group is this
|
benzyl group
|
|
|
draw a benzyl group
|
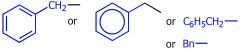
|
|
|
drawing of amines
|
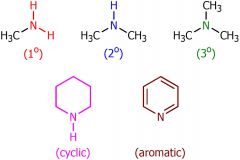
|
|
|
drawing of ethers
|
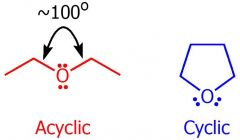
|
|
|
drawing of alcohols
|
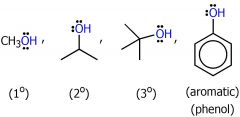
|
|
|
drawings of Alkyl halides/ halokalkanes
|
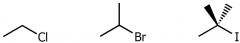
|
|
|
drawing of aldehydes and ketones
|
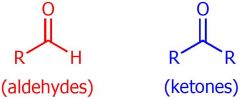
|
|
|
what are bonds of aldehydes
|
R-C-H - with O at top
|
|
|
what are the bonds of Ketones
|
R-C-R - with O at top
|
|
|
what are the bonds of AMines
|
R-NH2
|
|
|
what shape do aldehydes and Ketones have
|
trigonal planar (121-121-108)
|
|
|
what are bonds of carboxylic acids
|
R-OH with O at top
|
|
|
what are bonds of Esters
|
R-C-OR, wiht O at top
|
|
|
what are bonds of acid chlorides
|
R-C-Cl with O at top
|
|
|
waht are bonds of Amide
|
R-C-NR2, with O at top
|
|
|
what are bonds of acid anhydrides
|
C-O-C-R, o at top between O and R
|
|
|
what are bonds of Nitriles
|
R-C (triple bond) N
|
|
|
wave numbers are equal to what
|
1/wavelength in cm
|
|
|
what is wave number of O-H bond
|
3600-3200
|
|
|
what is wave number of N-H bond
|
3500-3200
|
|
|
what is the wave number of C-H bond
|
~3000
|
|
|
what is the wave number of Csp3-H bond
|
3000-2850
|
|
|
what is the wave number of a Csp2-H bond
|
3150-3000
|
|
|
what is the wave number of a Csp-H bond
|
3300
|
|
|
what is the wave number of a
c-(triple bond)-c |
2250
|
|
|
what is the wave number of a C-(tripplebond)-N
|
2250
|
|
|
what is the wave number of a C=O bond
|
1800-1650 (often 1700)
|
|
|
what is the wavenumber of a C=C bond
|
1650
|
|
|
what is the wave number of a benzene ring bond
|
1600-1500
|

