![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
76 Cards in this Set
- Front
- Back
|
Strong nucleophile/ weak base 1° |
SN2 |
|
|
Strong nucleophile/ weak base 2° |
Major SN2 Minor Sn1 |
|
|
Strong nucleophile/ weak base 3° |
Major Sn1 Minor E1 |
|
|
Strong nucleophile/ strong base 1° |
Major Sn2 Minor E2 |
|
|
Strong Nucleophile/ Strong base 2° |
Major E2 Minor Sn2 |
|
|
Strong nucleophile/ strong base 3° |
E2 |
|
|
Weak Nucleophile/ weak base 1° |
Sn2 E2 Unlikely to occur |
|
|
Weak nucleophile/ weak base 2° |
Sn1 Sn2 E1 E2 No reaction |
|
|
Weak nucleophile/ weak base 3° |
Sn1 E1 |
|
|
N3- |
Weak base/ strong nucleophile |
|
|
RCO2- |
Weak base strong nucleophile |
|
|
CN- |
Weak base strong nucleophile |
|
|
Cl- |
Weak base strong nucleophile |
|
|
HS- |
Weak base strong nucleophile |
|
|
H2S |
Weak base strong nucleophile |
|
|
Br- |
Weak base strong nucleophile |
|
|
RS- |
Weak base strong nucleophile |
|
|
RSH |
Weak base Strong nucleophile |
|
|
I- |
Weak base strong nucleophile |
|
|
ArO- |
Weak base strong nucleophile |
|
|
HO- |
Strong nucleophile strong base |
|
|
MeO- |
Strong base strong nucleophile |
|
|
EtO- |
Strong base strong nucleophile |
|
|
H2O |
Weak base weak nucleophile |
|
|
MeOH |
Weak nucleophile weak base |
|
|
EtOH |
Weak nucleophile weak base |
|
|
Good nucleophile |
Anions |
|
|
Right turn of priority of chirality |
R configuration |
|
|
Left turn of priority of chirality |
S configuration |
|
|
Cahn-Ingold-Prelog system priority |
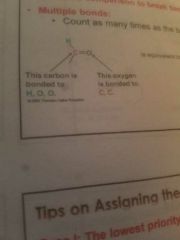
Greater atomic number the greater the priority Count as many times as bond order |
|
|
Achiral molecule |
Has plane of symmetry |
|
|
Chiral molecule |
No plan of symmetry |
|
|
Relative basicity of common bases |
OH<OR <C=_R <NH2 <CH=CH2 <CH2CH3 |
|
|
Relative acidity of conjugate acids |
H-OH>H-OR>H-C=_CR >H-NH2 >CH=CH2 >CH2CH3 |
|
|
Leaving group reactivity |
OH-, NH2-, OR- < F- < Cl- < Br- < I- < TosO- |
|
|
Increasing nucleophilicity in polar aprotic solvent (no available donating proton) |
I- < Br- < Cl- < F- |
|
|
Increasing nucleophilicity in polar protic solvent (with available donating proton) |
F- < Cl- < Br- < I- |
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|

