![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
13 Cards in this Set
- Front
- Back
|
Know the ten groups |

See pic |
|
|
Homologous series |
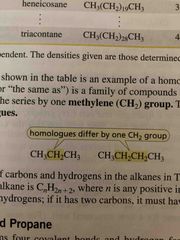
Group of compounds in which it differs by one methylene group (CH2) |
|
|
One possible structure |
Methane, ethane, and propane |
|
|
Constitutional isomers |
Have same molecular formula but differ in how atoms are connected Ie: butane is 4 C’s with an iso structural unit |
|
|
Alkyl groups |
Removing a H from an alkane replacing “ane” with “yl “ |
|
|
Alcohol amine and halide |
When a H is replaced by an OH: alcohol When a H is replaced with a NH2: amine When a H is replaced with a halogen: alkyl halide When replaced by a OR: ether |
|
|
2 alkyl 3-C groups |
Propyl: when H is removed from primary C of propane isopropyl: when H is removed from secondary C of propane |
|
|
4 four-C alkyl groups |
Butyl and isobutyl have H removed from primary C Sec-butyl:H is removed from secondary C Tert-butyl: H is removed from tertiary C |
|
|
G |
G |
|
|
Primary secondary tertiary carbons |
Primary is bound to one carbon Secondary is bound to 2 carbons Tertiary is bound to 3 carbons Note: a tertiary amine would also be bound to 3 carbons |
|
|
Favoring hydrophilicity |
Presence of ions Dipole moments Presence of van der waals: when comparing 2 non polar molecules, liquids larger will be more polar |
|
|
Lowest boiling point |
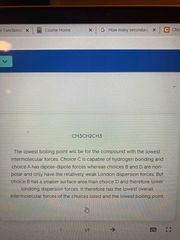
Choices are: Ch3Ch2Ch2Ch3 Ch3ch2ch2oh<—answer (Ch3)choh Ch3och2ch3 |
|
|
Learn these |

Pic |

