![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
17 Cards in this Set
- Front
- Back
|
AN ALPHA-HYDROXY ACID.
|
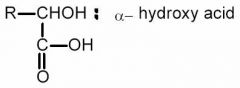
What is this?
|
|
|
What is the hybridization of the carbonyl carbon in N,N-dimethylformamide?
|
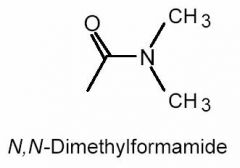
sp2;
- A carbonyl carbon is one that is double bonded to an oxygen. Since the carbon contains one π bond and three σ bonds (one double bond and two single bonds), it must be sp2 hybridized. - Note that we do not need to know the structure of N, N-dimethylformamide to answer this question. |
|
|
- The anion formed is stabilized by aromaticity.
- When 1,3-cyclopentadiene reacts with sodium, it goes from a nonaromatic to an aromatic compound. - A planar cyclic compound is aromatic if it satisfies Huckel's rule—that is, it has (4n+2) π electrons, where n is any integer. The product of the reaction has 6 π electrons, so is therefore aromatic. - An aromatic compound is very stable because the π electrons are delocalized throughout the ring. In this reaction the products are much more stable, so the reaction will be spontaneous. |
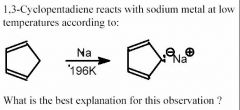
What is the BEST explanation for this observation?
|
|
|
When aminobenzoic acid loses a proton, the conjugate base assumes a charge of –1. Therefore, any resonance structure must also posses the same net charge.
|
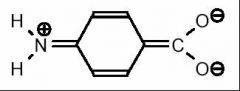
Why is this an appropriate resonsnce from of the conjugate base of p-aminobenzoic acid?
|
|
|
What are the characteristics of acetone; and why is it commonly used as a solvent to promote SN2 and E2 reactions?
|
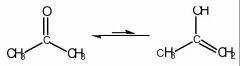
- It is aprotic and polar.
- Since it cannot form strong intermolecular bonds and is of low molecular weight it has a low boiling point. - Acetone exists in two forms, with the keto form predominating by 99%. This is because the carbon-oxygen double bond (the carbonyl bond) is much stronger than the carbon-carbon double bond. |
|
|
How many distinct organic compounds have the molecular formula C5H12?
|
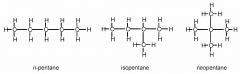
Three. Distinct compounds arise because of the different degrees of branching that can occur in the carbon chain.
|
|
|
For cyclic hexanes, what is the most favorable position for the substituents.
|
More favorable for substituents to occupy equatorial positions since they are more “out of the way.”
|
|
|
(T/F) Tautomers are a specific type of structural isomer.
|
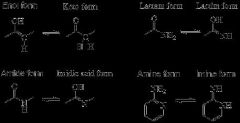
- True.
- This reaction results in the formal migration of a hydrogen atom accompanied by a switch of adjacent conjugated double bonds. |
|
|
What is the approximate C-N-C bond angle in diethylamine; and what is it's shape?
A. 107 deg B. 110 deg |
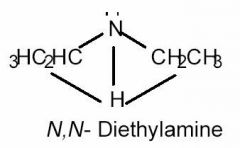
A
- The normal bond angle for an sp3 hybridized atom is 109.5 degrees. - By looking at the drawing of diethylamine, you will notice that nitrogen has a free electron pair. - Nonbonded (or lone) electrons pairs exert stronger repulsive effects, forcing the atoms attached to nitrogen down, and closer together. - VSEPR predicts that the bond angle MUST BE LESS than 109.5°. - THE SHAPE IS TRIGONAL PYRAMIDAL. |
|
|
- Bromine: axial; t-butyl: equatorial
- The t-butyl is the bulkiest substituent, so it must assume the equatorial position - Since the bromine is trans to the t-butyl and is two positions away, it must assume the opposite configuration as the t-butyl group, so is therefore axial. |
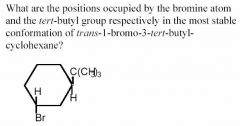
in terms of axial and equatorial for Bromine and t-butyl group?
|
|
|
- In order to identify the product, we start by recognizing and classifying the reactants.
Methyl chloride: primary alkyl halide Sodium ethoxide: strong nucleophile. - What reaction mechanism does this scream out? SN2 : the ethoxide attacks while the chloride leaves, forming dimethyl ether, choice C. (The sodium is just a spectator ion here). - This reaction type goes by the name of the Williamson ether synthesis. |
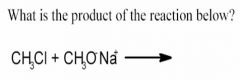
What is the reasoning behind this? What kind of reaction is this?
|
|
|
There are three chiral centers so 8 stereoisomers.
*** In general, be on the look out for meso compounds (not present in this scenario)! |
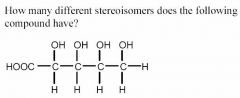
How many different stereoisomers does the following compound have?
|
|
|
Which of the following compounds will exhibit the greatest dipole moment: (E)-1,2-Dichloro-1,2,-diphenylethene AND (Z)-1,2-Dichloro-1,2-diphenylethene?
|
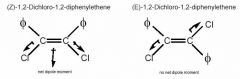
(Z)-1,2,Dichloro-1,2-diphenylethene
|
|
|
How many structural isomers of C3H6Br2 are capable of exhibiting optical activity?
|

see above.
|
|
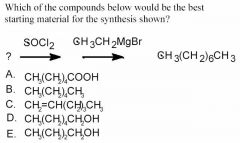
see above.
|
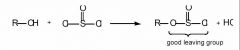
- This is a SN2 reaction made to look difficult.
- SOCl2 reacts with primary and secondary alcohols to generate something with a very good leaving group, ClSO2–. (OH– is a poor leaving group.) - The compound formed is reactive in nucleophilic substitution reactions. - The Grignard reagent is a good nucleophile. To get the question correct, you have to identify R. Since our product has 8 carbons, and 2 came from the ethyl magnesium bromide, our starting compound must have 6 carbons attached to a leaving group; answer D. |
|
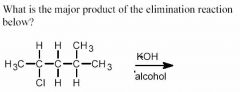
See above.
|
- KOH dissociates in to K+ and OH– ions, the latter of which is a strong base. The elimination will therefore proceed via the E2 mechanism. Since the hydroxide ion is not a bulky base, and the substrate is a secondary alkyl halide (rather than a more sterically hindered tertiary alkyl halide), the resulting alkene will be the more thermodynamically stable, more substituted one depicted above.
- I.e. the double bond forms between the second and third carbon atoms. |
|
|
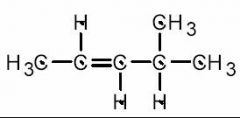
A SINGLE ALKENE PRODUCT IS FORMED AND N-PROPYL CHLORIDE IS PRESENT IN EXCESS
*** (there is no such thing as racemic and inversion when it comes to elimination reactions!!!) - When either of the alkyl chlorides undergoes elimination, the same alkene will be formed: propene. - Since this is an E1 reaction, the substrate that can form the more stable carbocation will be most reactive. - Isopropyl chloride, a secondary alkyl halide, can form a more stable carbocation than n-propyl chloride, a primary alkyl halide. ==> Therefore isopropyl chloride will react the most and n-propyl chloride will be present in excess. |
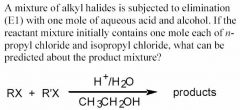
see above
|

