![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
114 Cards in this Set
- Front
- Back
|
keys to optimal drug delivery
|
right drug
right amount right time right location right patient |
|
|
oral formulations account for _____% of drug dosages
|
84%
|
|
|
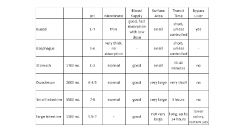
Stomach:
Volume pH fasted pH fed |
1.6 L
1.7 5 |
|

|
|
|

|
|
|
|
segregation of particles
|
small particles will sink to the bottom (like mixed nuts)
|
|
|
hard gelatin capsules are filled with:
|
- solids
- powders - granular or pelletized materials - oils |
|
|
soft gelatin capsules are filled with:
|
- semisolids
- liquids - granular or pelletized materials |
|
|
alternatives to gelatin capsules
|
1. Pullulan- a water soluble polysaccharide
2. HPMC- a water soluble polymer |
|
|
most common oral dosage form
|
tablet
|
|
|
best way to make a drug more soluble
|
make the particles as small as possible
|
|
|
types of tablet coating
|
1. sugar coating
2. film coating 3. enteric coating |
|
|
disadvantages of tablets
|
- inflexible dosage form
- not prepared extemporaneously - large no. of ingredients - dissolution of drug - drugs sensitive to moisture, oxygen, light - what is pharmacist's responsibility in regard to tablets? |
|
|
MCT
|
multiple compressed tablet
-layered tablets, press-coated tablets, inlays |
|
|
DT
|
dispensing tablet
|
|
|
advantages of buccal and sublingual tablets
|
- rapid onset
- avoid 1st pass metabolism - avoid gastric fluids |
|
|
binder
|
granules which help the formulation flow
|
|
|
implants
|
act as a resevoir for the drug
|
|
|
disintegraters
|
cause tablet to break up
|
|
|
main problem with lubricants
|
all lubricants are insluble in water and and ruin absorption or look unappealing
|
|
|
common flow problems
|
- non-uniform flow disrupting uniformity
- uneven flow cause segregation of active ingredients - need for force feeders, vibrators, custom hoppers, etc. - non reproducible manufacturing operations - low process yields due to inefficient material handling & high reject levels |
|
|
Basic tablet manufacturing processes
|
1. direct compression
2. wet granulation 3. dry granulation |
|
|
direct compression suitable for drugs which:
|
- drugs that have large doses
- drugs that are free flowing and crystalline - drugs that have cohesive properties |
|
|
advantages of direct compression
|
- simplicity and time
- no prior granulation - avoid moisture and heat |
|
|
disadvantages of wet granulation
|
- usually needs a solvent that needs to be removed
- more complicated process |
|

|
capping
|
|

|
lamination
|
|

|
a- picking
b- stress cracking |
|

|
chipping
|
|
|
Purposes of tablet Coating
|
1. Enhance palatability
2. Increase stability of active drug 3. Increase mechanical integrity of tablet 4. Enhance elegance 5. Protect hands and clothes from staining 6. Modify drug release profile 7. Avoid side effects (i.e. gastric irritation) 8. Avoid incompatibility 9. Identification of product |
|
|
rate of dissolution
|
the amount of drug substance that goes in solution per unit time under standard conditions of liquid/solid interface, temperature, and solvent composition
|
|
|
difference between solubility and dissolution
|
solubility- how much can dissolve
dissolution- the process of dissolving |
|
|
dC/dt
|
dissolution rate (change in solute concentration per unit time)
|
|
|
A
|
the effective surface area of the solid being dissolved
|
|
|
Cs
|
concentration of solid in the boundary layer
|
|
|
Ct
|
concentration of solid in the medium
- usually ignored (sink conditions) |
|
|
k1
|
new dissolution rate constant
|
|
|
D
|
diffusion coeffficient of the solid
|
|
|
V
|
volume of the dissolution medium
|
|
|
h
|
thickness of the diffusion layer
|
|
|
w
|
mass of drug remaining to dissolve at time t
|
|
|
polymorphism
|
when a substance exists in more than one crystalline form
|
|
|
stable polymorph has _________ energy state, ___________ MP, and _________ aqueous solubility than metastable polymorph
|
lower energy state
higher MP lower aqueous solubility |
|
|
what happens to a metastable polymorph when it precipitates out of solution?
|
It will change into the stable polymorph
|
|
|
Dissolution testing apparatus (2)
|
Apparatus I:
- rotating basket for tablets Apparatus II: - paddle for tablet, capsules, modified drug products |
|
|
Components of Fasted State Simulated Intestinal Fluid (FaSSIF)
|
- Sodium taurocholate
- Lecithin - Na OH pellets - NaH2PO4H2O - NaCl - purified water |
|
|
Components of FeeSSIF)
|
- Sodium taurocholate
- Lecithin - NaOH pellets - Glacial Acetic Acid - NaCl - Purified water |
|
|
f2
|
similarity factor used in dissolution profile comparison
- when two profiles are identical, f2 = 100 - an average difference of 10%, f2 = 50 |
|
|
API standard for Bioequivalence
|
If dissolution in vivo and permeability are the same, then we can assume that metabolism in GI-wall, metabolism, and excretion will also be the same.
|
|

|

|
|
|
Biopharmaceutics Class I:
- RLS - IVIVC - when is dissolution not likely to be rate limiting? - Examples (3) |
HS/HP
RLS: Gastric Emptying IVIVC: Correlation if dissolution rate < gastric emptying; otherwise no correlation When dissolution rate > gastric emptying, dissolution is not likely to be rate limiting Ex: Metoprolol, Verapamil, Propanolol |
|
|
Biopharmaceutics Class II:
- RLS - IVIVC - Examples (3) |
LS/HP
RLS: Dissolution IVIVC: Yes, if in vitro dissolution rate is similar to in vivo dissolution rate, unless dose is very high Ex: Naproxen, Carbamazepine, Ketoprofen |
|
|
Biopharmaceutics Class III:
- RLS - IVIVC - Examples (3) |
HS/LP
RLS: Permeability IVIVC: No Ex: Atenolol, Cimetidine, Ranitidine |
|
|
Biopharmaceutics Class IV:
- RLS - IVIVC - Examples (2) |
LS/LP
RLS: varies (In vitro dissolution may not be reliable) IVIVC: Limited or none Ex: Furosemide, Hydrochlorothiazide |
|
|
FDA requirements for BE study waiver
|
- BCS Class I
- absorption > 90% - highest dose soluble in <250 ml pH 1-7.5 - dissolution in vitro >85% in 30 min - f2 dissolution profile similarity test - only well established excipients - not drug with narrow therapeutic window |
|
|
IVIVC
|
In-vitro in-vivo correlation
- mathematical model describing the relationship between in vitro and in vivo properties of drug |
|
|
Methods to achieve IVIVC
|
- pharmacological correlation
- Semi quantitative correlation - Quantitative correlation |
|
|
Levels of IVIVC Correlation
|
- A
- B - C - multiple level C |
|
|
Level A Correlation
|

|
|
|
Level B Correlation
|

|
|
|
Multiple Level C Correlation
|

|
|
|
Steps to develop an IVIVC Model
|
1. Use 2 formulations with ≥ 2 release rates (≥3 recommended)
2. Attain in vitro dissolution profiles 3. Determine in vivo plasma conc. as a function of time 4. Estimate in vivo drug release rate via deconvolution 5. Correlate % absorbed (in vivo data) as a function of % dissolved (in vitro data) |
|
|
in vivo data
|
% absorbed
|
|
|
in vitro data
|
% dissolved
|
|
|
Advantages of Buccal Delivery
|
- mucosa is well supplied with both vascular and lymphatic
drainage; first-pass metabolism avoided - patient acceptance - control and manipulation of drug permeation - delivery of orally inefficient drugs and peptides |
|
|
Disadvantages of Buccal Delivery
|
- inconvenience
- swallowed drug is subject to first pass metabolism - limited to small doses |
|
|
Buccal-
Sublingual- Gingival- Palatal- |
Buccal- cheek
Sublingual- ventral tongue; floor of mouth Gingival- gums surrounding teeth Palatal- roof of mouth |
|
|
Potential losses of Buccal drug delivery
|
- swallowing
- dilution by saliva - protein binding - metabolism - absorption in other oral regions |
|
|
Methods to enhance cell permeability of drugs
|
- organic solvents (toluene, DMSO, phenethyl alcohol)
- chelating agents (EDTA) - surfactants, detergents - lysolecithin -antibiotics - proteins |
|
|
Striant
|
Buccal system for testosterone replacement
|
|
|
Advantages of Conventional Oral Delivery
|
- convenient
- cheap - variety of dosage forms |
|
|
Disadvantages of Conventional Oral Delivery
|
- inefficience due to partial absorption
- first pass effect - effect of food & GI motility on absorption - local effect (i.e. antibiotics kill GI flora) - patient must be able to swallow dosage |
|
|
Methods of crossing membranes
|
1. diffusion through channels
2. dissolving in lipid phase + soluble in aqueous phase 3. carrier-mediated transport |
|
|
At same pH, acids with _______ pKa values are absorbed less, whereas bases with ______ pKa values are absorbed more
|
lower; lower
|
|
|
At same pKa, acids are absorbed more in ______ pH environment, whereas bases are absorbed more in _______ pH environment.
|
lower pH; higher pH
|
|
|
Classic Paradigm of Cyclosporine
____% loss to feces ____% metabolized by liver ____% to systemic circulation |
65% feces
8% liver 27% systemic |
|
|
New Paradigm of Cyclosporine
____% loss to feces ____% metabolized by gut wall ____% metabolized by liver ____% to systemic circulation |
14% feces
51% gut wall 8% liver 27% systemic |
|
|
Efflux Pump
Examples (2) |
protein pumps which transport molecules out of the cell
- PGP, cytochrome P450 3A |
|
|
Effect of polymorphs on solubility and bioavailability
|
in most cases, no major changes
***we do need to know when it is important*** |
|
|
Chloramphenicol Form B
|
form B is more bioavailable, but less stable
- can effect shelf life as B morphs into more stable, less soluble A |
|
|
Dissolution rate of an anhydrous phase
usually is ______ than that of the hydrate Exception: |
higher
Exception= erythromycin |
|
|
Methods to increase dissolution
|
- reduce particle size
- oral solution - wetting agents - salt form - lipid solubilization - prodrug |
|
|
Use of relationship between Half-life and Serum creatinine level of drug
|
can use to adjust dosage for a patient
|
|
|
Effect of__________ on gastric emptying
- large glass of water - large meal - small snack |
water: fast emptying
large meal: slow emptying small snack: slow emptying |
|
|
Estimated transit times:
50% of stomach Total emptying of stomach 50% of small intestine transit through colon |
50% of stomach 2.5-3 hr
Total emptying of stomach 4-5 hr 50% of small intestine 2.5-3 hr transit through colon 30-40 hr |
|
|
The _______ the stomach emptying the higher the plasma concentration.
|
quicker -> higher plasma conc.
|
|
|
Griseofulvin
|
a drug that's absorption is improved by presence of fatty food (exception)
- apparently the poorly soluble drug is griseofulvin is dissolved in the fat and then more readily absorbed |
|
|
Physiochemical Barriers to Oral Drug Delivery
|
- chemical degradation
- thermodynamics - partitioning, extent and rate - absorption effects - molecular size - solubility/dispersability |
|
|
Biological Barriers to Oral Drug Delivery
|
- bacterial degradation
- enzymatic degradation - first pass - immunological (GI associated immunities) |
|
|
Physiological variables affecting Oral Drug Delivery
|
- pH of GI tract
- membrane permeability - site specific absorption - gut wall metabolism - blood flow - GI motility - luminal contents - gastric/intestinal secretions |
|
|
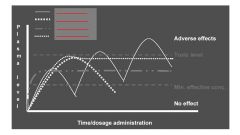
Benefits of Oral Sustained Release
|
- increased patient compliance (reduced frequency of dosing)
- sustained pharmacodynamic response - reduced side effects (fewer peaks/valleys) - enhanced bioavailability |
|
|
LA
|
Long Acting
|
|
|
SA
|
Sustained Action
|
|
|
SR
|
Sustained Release
|
|
|
TR
|
Time Release
|
|
|
ER
|
Extended Release
|
|
|
"Magic Bullet"
|
Drug selectively directed to site of action -> no side effects
|
|
|
Stealth Concept
- method |
a PEG coated liposome is not seen by defense mechanisms in the blood, hence it successfully carries drug molecules to the target cells
-by controlling size, you can direct where molecule will leave blood vessels |
|
|
Monolithic system
|
tablets
|
|
|
Multiparticulate System
|
granules or pellets in capsules
- can use two or more types of beads to get multiple delivery rates |
|
|
Mechanisms of Controlled Release
in Oral Delivery Systems |
- Diffusion
- Solvent-activated release - polymeric degradation |
|
|
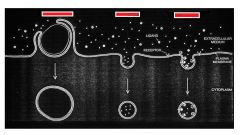
Diffusion Controlled Release System
|
- drug is either encapsulated in a polymer membrane or suspended within a polymer matrix
- water diffuses into the membrane or matrix, drug dissolves, then drug diffuses out of the polymer |
|
|
Solvent Activated System
|
-An osmotic agent draws water into the membrane through a small hole
- the drug is then forced out through the hole due to increased pressure |
|
|
OROS
|
an osmotic control system
|
|
|
Polymeric Degradation
|
-drug is contained within a polymer membrane or matrix
- polymer degrades and releases drug at specific location |
|
|
Degradation Mechanism A
|
water-soluble polymers are made insoluble by cross-linking them together.
When the cross-links are broken at some point in the body, the polymer will dissolve. |
|
|
Degradation Mechanism B
|
water-insoluble polymers are made soluble by hydrolysis or ionization of side groups.
|
|
|
Degradation Mechanism C
|
involves the use of insoluble polymers that are cleaved into soluble monomers
|
|
|
Responsive Drug Delivery System
|
stays inactive in the body until a person is exposed to danger
- i.e. insulin pumps |
|
|
Why are drugs good or bad candidates for
oral delivery? - HIGH DOSE • Good: Better for manufacturer • Bad: Inconvenient for patient -LONG HALF-LIVES • Good: less frequent administration • Bad: Increased duration of side effects - VERY POTENT DRUGS • Good: good bioavailability • Bad: Hard to ensure content uniformity - POORLY WATER SOLUBLE COMPOUNDS • Good: Useful for controlled (extended release formulation) • Bad: Poor dissolution can lead to poor bioavailability - NOT GOOD AT ALL • Irregularly or poorly absorbed drugs • Drugs that do not exhibit a relationship between plasma levels and biological activity - Of course exceptions will occur |
-
|
|

|

|
|

|

|
|
|

|
|
|

|

