![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
69 Cards in this Set
- Front
- Back
|
List 7 methods of drug adminstration |
1. Oral 2. Rectal 3. Subcutaneous 4. Intravenous 5. Intramuscular 6. Transdermal 7. Pulmonary |
|
|
Define therapeutic effect |
Desired biological change that cures or alleviates illness, caused by a drug . |
|
|
Define a placebo and describe the placebo effect |
A biologically inert substance that assists the body's natural healing process. The therapeutic effect of a placebo is the placebo effect. |
|
|
Define double-blind test |
Drug trial where neither the researchers directly observing the patients nor the patients themselves know who is given the real drug or placebo. |
|
|
Define side-effect |
Unwanted effects of a drug that do not contribute to the therapeutic effect. |
|
|
Define TI |
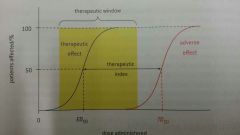
Therapeutic index.
The ratio between toxic / lethal dose and effective dose.
TI = TD50 / ED50
The greater the TI, the safer the drug. |
|
|
Define TD50 |
Toxic Dose The dosage of drug that causes a toxic effect in 50% of test subjects |
|
|
Define LD50 |
Lethal Dose The dosage of drug that kills 50% of test subjects |
|
|
Define ED50 |
Effective Dose The dosage of drug that causes therapeutic effect in 50% of test subjects |
|
|
Define therapeutic window |
The range of drug doses that causes therapeutic effects. Defined by minimum drug dosage required to cause therapeutic effect, and minimum dosage required to cause toxic effects. |
|
|
Define drug availability |
The fraction of administered drug absorbed into bloodstream |
|
|
Define drug tolerance |
The progressively high drug doses users require to obtain the same therapeutic effects. |
|
|
Define drug addiction |
Compulsive desire of the user to take a drug regardless of the adverse effects it might cause. |
|
|
Outline drug action |
Drugs interect with enzymes or cell receptors. Drugs are often inhibitors. |
|
|
Outline the stages of drug development |
1. Drug discovery - identification of lead compound - New chemical entity (NCE)
2. Drug design - Alternative / complementary approach to find lead compound
3. Pre-clinical trials - similar compounds to lead compound are synthesised - most effective, safe, and accessible compound selected for clinical trials
4. Clinical trails Drug is tested on humans, in a double-blind experiment. Phase I Small number of healthy volunteers are tested for TD50 and side effects Phase II Small number of patients are tested for ED50 and side effects Phase III Large number of patients are tested to comparee with other drugs. Further data on effectiveness and side effects.
5. Post-clinical studies / Phase IV Long term study |
|
|
Outline the synthsis of aspirin |
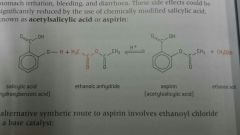
1. Salicyclic acid is isolated from willow bark 2. Salicyclic acid is reacted with ethanoic anhydride to form aspirin and ethanoic acid (addition-elimination reaction) |
|
|
Explain the effects of aspirin |
1. Mild analgesic 2. Antipyretic 3. Anticoagulent 4. Non-narcotic analgesic 5. Non-steroidal anti-inflammatory drug (NSAID) - supresses prostaglandin production - in turn decreases thromboxin production |
|
|
List the risk of taking aspirin |
Stomach ulceration - aspirin synergises with ethanol and other anticoagulants |
|
|
Explain how aspirin's bioavailability is increased |
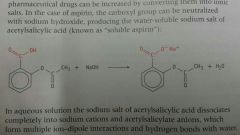
1. Aspirin is almost insoluble in water 2. Asirin is converted to a salt (using NaOH) |
|
|
Outline the mechanism of action of penicillin |
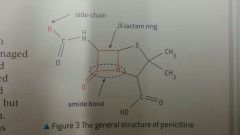
- 90 degree bond angles in beta-lactam ring creates ring strain - penicillin reacts with transpeptidase, weakening cellulose cross-linkages in bacterial cell walls - osmotic pressure within bacteria during mitosis causes lysis |
|
|
Explain why morphine has difficulty crossing the blood-brain barrier, and how the barrier can be overcome |
Morphine has 1 amino and 2 hydroxyl groups, causing it to be polar, so has difficulty crossing the lipophilic cell membranes that coat blood vessels. A OH group in morphine is replaced with a OCH3 to form codeine, which is less polar, and is therefore more bioavailable. Both OH groups in morphine are replaced with OCOCH3 (ester) groups, forming diamorphine with reduced polarity. |
|
|
State the function of antacids and provide an example |
Antacids react with HCl in gastric juice, increasing pH. Aluminium hydroxide Calcium carbonate Sodium Hydrogen Carbonate |
|
|
Explain how gastric acid is produced int he stomach, and give example of drugs that prevent pH from becoming too low |
Acid secretion in stomach occurs when histamine binds to H2-histamine receptors on gastric lining cells. H+ ions are actively pumped into stomach by the gastric proton pump. Drug rantidine is a H2-receptor antagonist (temporary) Drugs omeprazole and esomeprazole are gastric proton pump inhibitors, and reduce H+ production (prolonged inhibition) |
|
|
Explain the acid-base buffer system in the human body |

|
|
|
Briefly outline the 4 different mechanisms in which antiviral drugs act on viruses
|
1. Prevents virus from attaching to host cell surface 2. Prevents viral uncoating, so viral genetic material cannot be injected into host cell 3. Inihibits reverse transcriptase, so viral genetic material is not replicated 4. Inhibits the release of viruses from host cell after reproduction |
|
|
Outline how amantadine and rimantadine are effective antiviral drugs |
Both drugs inhibit the uncoating of virus strains, so they cannot inject genetic material into host cell. |
|
|
Outline how acyclovir and zidovudine are effective antiviral drugs |
Both drugs undergo phosphorylation in the host cell and produce non-standard nucleotides, which are incorporated into DNA. Enzymes formed by the DNA is inactive and cannot replicate viral components. |
|
|
Outline how oseltamivir and zanamivir are effective antiviral drugs |
Both drugs inhibit enzymes neuraminidases, which trigger viral budding, trapping viruses within host cell. |
|
|
Define HIV. |
Human Immunodeficiency Virus Type of virus that causes Acquired Immunodeficiency Sydrome. |
|
|
Define AIDS |
Acquired Immunodeficiency Syndrome Progressive failure of the immune system, may lead to life-threatening infections. |
|
|
Explain how zidovudine is an effective drug for HIV |
Drug inhibits reverse transcriptase enzymes. |
|
|
List 5 reasons that caused increase in antibiotic resistance over time |
1. Over-prescription of antibiotics 2. Non-compliance of patients in finishing antibiotic course 3. Use of antibacterials in agriculture 4. Release of antibacterial waste by hospitals and pharmaceutical industry 5. Bacteria replicate at a very fast rate, and may develop resistance (e.g. penicillinase) due to mutations |
|
|
Explain how LLW are disposed of and give examples of LLWs |
Low-Level Waste have short half-lives, and are stored in shielded containers until radiation level drops below safe limit, then stored in underground repositories. Examples of LLWs are Co-60 and Cs-137 |
|
|
Explain how HLW are disposed of. |
High-Level Waste have long half-lives and produce much heat due to nuclear reactions. They are cooled using water, and are either vitrified or converted to synthetic rock, and stored in underground repositories. |
|
|
List 3 organic solvents and outline the risks of using them. |
1. Benzene 2. Chloroform 3. Ethanol 1. Benzene and Chloroform increase risk of cancer 2. Many organic solvents are flammable (ethanol) |
|
|
List 3 different chlorinated solvents, and explain how they negatively impact the environment. |
1. Carbon tetrachloride 2. Chloroform 3. Dichloromethane 1. Ozone-depleting agents 2. Non-biodegradable and accumulate in water bodies |
|
|
State the primary goal of green chemistry and outline how it is achieved. |
Reduce the environmental impact of technological processes by minimizing the use and generation of hazardous chemicals. 1. Aqueous / solvent-free reactions 2. Renewable starting materials 3. Mild reaction conditions 4. Ultilization of by-products 5. Increase atom economy 6. Decrease number of reaction steps |
|
|
Give and example of the impact of biotechnology on green chemistry |
Shikimic acid is a precursor to oseltamivir. Its extracted from Chinese star anise was a year-long process. It also produced unsafe lithium hydride. Biotechnology allowed Shikimic acid production at an industrial scale through genetically modified E. coli. |
|
|
Taxol |
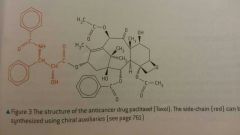
- Extracted from pacific yew tree
|
|
|
Docetaxol |
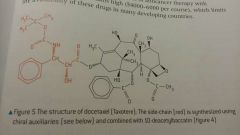
- More active than Taxol - More water soluble |
|
|
Explain the mechanism of chiral auxillaries |

|
|
|
Define prophylactic |
A drug taken for preventative healthcare, to prevent an illness from occuring. |
|
|
Briefly explain how aspirins in purified |
1. Aspirin is dissolved in hot solvent (ethyl ethanoate / ethanol) to form a near-saturated solution 2. Insoluble impurities are removed through vacuum filtration 3. Solution is allowed to cool. Bottom of flask may be scratched to initiate crystallization. 4. Steps 1-3 are repeated for higher purity. |
|
|
How is the purity of aspirin determined? |
1. Gas chromatography 2. Melting point 3. Infrared spectrum |
|
|
Briefly explain how opiates are strong analgesics.
|
Opiates such as morphine and codeine bind to opioid receptors in the brain, blocking pain signals from reaching the brain. |
|
|
Explain how dependence on opiates can be managed
|
1. Gradual reduction of opiate dosage 2. Prescription of methadone, which binds to opioid receptors for prolonged period of time |
|
|
Define indigestion |
The irritation of stomach lining due to excess acid, producing pain or discomfort in upper abdomen |
|
|
Define heartburn (acid reflux). |
The rising of stomach acid into oesophagus, causing a burning sensation. |
|
|
Define peptic ulcer |
Erosion of gut lining caused by acid penetrating mucous layer. |
|
|
Explain the therapeutic effects omeprazole |
Omeprazole is a gastric proton pump inhibitor. it prevents H+ ions from being actively pumped into stomach cavity. |
|
|
Explain the therapeutic effects of esomeprazole |
Esomeprazole is a gastric proton pump inhibitor. it prevents H+ ions from being actively pumped into stomach cavity. |
|
|
Explain the therapeutic effects of rantidine |
Rantidine is a H2-receptor antagonist, and prevents histamine from binding to receptors on stomach cells, and so H+ ions are not produced. |
|
|
State the function of hemagglutinin (HA). |
Hemagglutinin allows viruses to bind to host cell surface receptors. It binds to sialic acid residues on glycoproteins. |
|
|
State the function of neuraminidase (NA). |
Neuraminidase is an enzyme that hydrolyses (breaks) the bond between sialic acid residue and glycoprotein. |
|
|
Explain how paclitaxel is an effective drug |
Paclitaxel is used during chemotherapy as it bind to microtubules in cells, preventing cell division from occuring completely. |
|
|
Explain how enantiomers can be distinguished from one another. |
1. A polarimeter can be calibrated with pure solvent 2. First enantiomer is placed into polarimeter, and polarising filter is rotated until its light is parallel to the enantiomer 3. The same is performed with second enantiomer 4. Direction and angle in which polarised light rotate is recorded |
|
|
Give an example of alpha-decay. |
226,88,Ra -> 222,86,Rn + 4,2,He |
|
|
Give an example of beta-decay. |
12,5,B -> 11,6,C + 0,-1,e |
|
|
Define half-life |
The time required for the number of radioactive nuclei present in a sample at any given time to fall to half its values. |
|
|
1st Rate equation for radioactive decay |
A = lamda x N A: Activity / becquerel (Bq) lamda: decay constant N: Number of nuclei present Parallel to rate equation: rate = k [X] |
|
|
2nd Rate equation for radioactive decay |
N = No e^(-lamda t) No: Initial number of undecayed nuclei N: Number of undecayed nuclei at time t lamda: Decay constant |
|
|
Name 2 radioisotopes used in medicinal tracing |
technium-99 emits gamma rays flourine-18 emits beta particles |
|
|
Briefly outline how ionizing radiation can harm the body |
Ionizing radiation cause direct ionization of DNA, changing their structure. It can also cause formation of free radicles (H2O -> H. + OH.), which interacts with DNA, damaging it. |
|
|
Outline the difference in functional groups between morphine, codeine and diamorphine |
Morphine: Two OH groups Codeine: One OH, one OCH3 Diamorphine: Two OCOCH3 |
|
|
Discuss difficulties in solving AIDS |
1. HIV mutates quickly 2. Contraception is not practised in many situations 3. Stigma and discrimination against HIV patients deter treatment 4. Lack of government support and funding in healthcare 5. Health literacy rates are low |
|
|
Explain why technium-99 is a common isotope in nuclear medicine |
1. Technium-99 releases photons with X-ray wavelength, and can be detected easily 2. Emitted photons have low energy, and is therefore safe 3. Readily forms complexes with ligands, and can be injected into body |
|
|
Outline why yttrium-90 is a common radioisotope in nuclear medicine |
Emits beta particles |
|
|
Outline why lutettium-177 is a common radioisotope in nuclear medicine |
Emits both beta and gamma particles. Short penetration length. |
|
|
State the chemical changes within a breathalyzer |
Potassium dichromate Orange (anhydrous) Green (hydrated) |

