![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
16 Cards in this Set
- Front
- Back
|
Describe principle of operation of batteries |
Chemical energy of a compound acts as a storage medium During discharge, a chemical process converts the energy into electricity |
|
|
Name and describe the two kinds of batteries |
Primary-convert Chen to elec once Secondary-rechargeable, reversible converters for multiple charges and discharges |
|
|
Draw a basic primary battery |
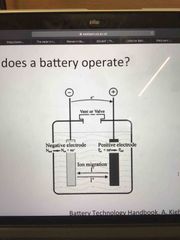
Back (Definition) |
|
|
What happens at the negative electrode (cathode) |
Cations flow to it |
|
|
Describe the two structural types of batteries |
Wet cells-electrolyte is a liquid like an am solution and dry cells-electrolyte is a solid |
|
|
Draw a typical wet cell |
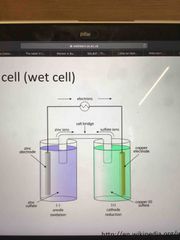
Back (Definition) |
|
|
Draw and describe a dry cell |

Back (Definition) |
|
|
Why might batteries leak |
1)outer casing corrodes, weakening the seal points 2) at discharge, form an solutions and gases which causes pressure build up, so seal points can fail |
|
|
What rechargeable batteries have the highest efficiency |
Lithium ion |
|
|
What is a lithium ion battery made up of |
Lithium oxide (positive electrode) and graphite (negative electrode) and lithium salt (electrolyte) |
|
|
What must the positive electrode materials be? Give examples |
Readily reducible/oxidisable Must react with Ali reversible and rapidly without structural changes Lithium cobalt oxide, lithium nickel manganese oxide, lithium iron phosphate. |
|
|
What must the negative electrode material be |
Litigated graphite !! |
|
|
Give the equations at the electrodes in lithium ion batteries |

’Negative’ (Cos ions that go to it are -ve, tbh should make it ‘+ve’ since that would make sense to attract them, but acc the additional negatives just make it -ve too. (anode) get reduction Positive (cathode) get oxidation |
|
|
What lithium salts make good electrolytes |
LiPF6 LiBF4 |
|
|
Research into new metal oxides for anode and cathodes focuses on improving what? |
Increasing reversibility Faster charging times Low cost Environmental friendliness |
|
|
Cc |
Cc |

