![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
20 Cards in this Set
- Front
- Back
|
pKa of alcohol
R-OH |
16
|
|
|
pKa of ethers
R-O-R |
50
|
|
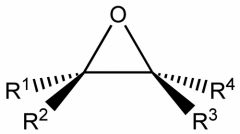
|
epoxide (or oxane)
|
|
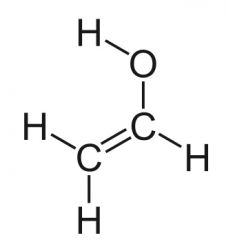
|
vinyl alcohol
-enols |
|
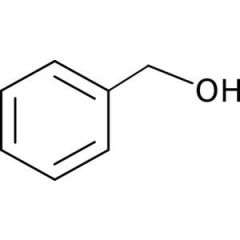
|
aromatic alcohol
-phenols pKa: 10-11 |
|
|
-OH group is called
|
hydroxy group
|
|
|
naming alcohols
|
suffix: -ol
indicated where attached before the suffix EX: 4-methylpent-2-ol (new IUPAC) |
|
|
naming ethers
|
name both sides with ether at the end
EX: CH₃-O-CH₂-CH₃ = ethyl methyl ether |
|
|
naming epoxides
|
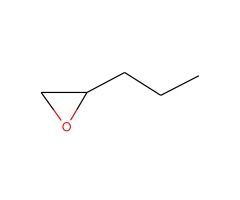
prefix: epoxy- (like a substituent)
1,2-epoxypentane |
|
|
Alcohol and BP
|
the higher the surface area the higher the BP
- primary alcohol higher BP than secondary, which is higher than tertiary. |
|
|
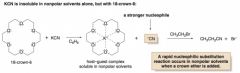
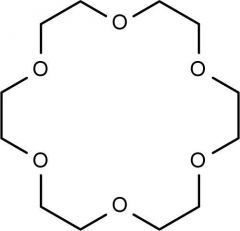
Crown Ether
- used to speed up SN2 reactions b/c they trap cations [Na⁺, Li⁺, K⁺, Ca⁺] |
|
|
alcohol synthesis
|
R-X + [KOH, NaOH, LiOH] --> R-OH
(potassium hydroxide, sodium hydroxide, lithium hydroxide) Alcohols and ethers are both common products of nucleophilic substitution. They are syn- thesized from alkyl halides by SN2 reactions using strong nucleophiles. As in all SN2 reactions, highest yields of products are obtained with unhindered methyl and 1° alkyl halides. |
|
|
Williamson Ether Synthesis
|
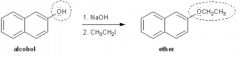
1. Start with something that has an alcohol functional group, remove the H from the -OH (with a strong base like NaH).
2. Then add the least sterically hindered alkyl halide (like CH₃I), which will loose its halogen to the Hydrogen and the methyl group will attach to the Oxygen. |
|
|
Ether Cleavage
|
generates an alkyl halide and a biproduct (2 steps)
|
|
|
preparation of alcohols
|

• The mechanism is SN2.
• The reaction works best for CH3X and 1° RX. |
|
|
preparation of alkoxides
|
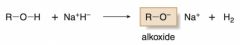
|
|
|
Le Châtelier’s principle
|
a system at equilibrium will react to counteract any disturbance to the equilibrium
|
|
|
pyridine
|
the base that removes a β proton during elimination (often used with POCl₃, which is used in place of a strong acid to create a better leaving group)
|
|
|
What is used for alcohol dehydration?
|
- H₂SO₄ or TsOH
- POCl₃ with pyridine (base) |
|
|
SOCl2 and PBr3
|
1° and 2° alcohols can be converted to alkyl halides using these.
• SOCl2 (thionyl chloride) converts alcohols into alkyl chlorides. • PBr3 (phosphorus tribromide) converts alcohols into alkyl bromides. Both reagents convert –OH into a good leaving group in situ—that is, directly in the reaction mixture—as well as provide the nucleophile, either Cl– or Br–, to displace the leaving group. |

