![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
27 Cards in this Set
- Front
- Back
|
What two kinds of substituents must you have on the aromatic compound in order for SNAr to proceed?
|
A leaving group (halogen) and an EWG
|
|
|
Why must the aromatic compound have an EWG?
|
The ring must be e- deficient in order to stabilize the extra charge
|
|
|
How is the rate of displacement impacted by halogen?
|
Fluorine is far faster than everything else.
|
|
|
Where should the EWG be placed in relation to the halogen, ideally?
|
o/p
|
|
|
What is the rate limiting step of SNAr?
|
Addition of the nucleophile
|
|
|
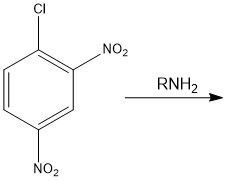
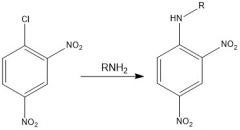
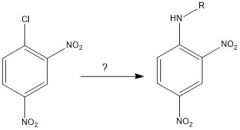
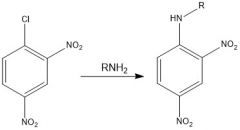
Draw the overall scheme of SNAr
|
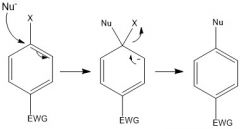
|
|
|
What is the SNAr intermediate called?
|
Meisenheimer
|
|
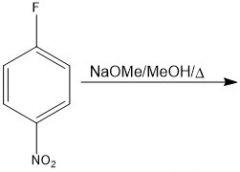
|
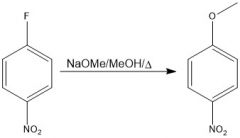
|
|
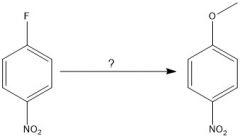
|
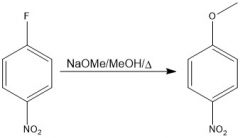
|
|
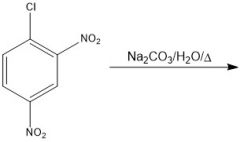
|
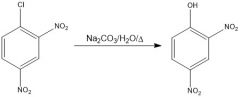
|
|
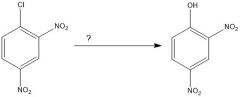
|
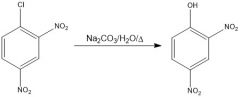
|
|
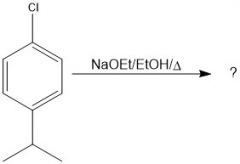
|
N.R.
|
|
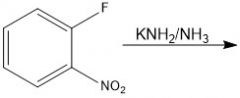
|
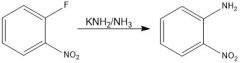
|
|
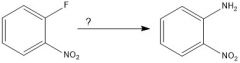
|
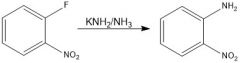
|
|
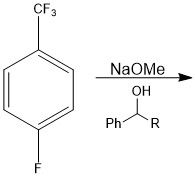
|
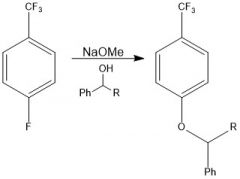
OH is a bad nucleophile, so NaOMe is there to deprotonate it
|
|
|
|
|
|
|
|
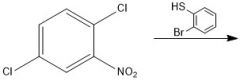
|
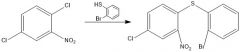
|
|
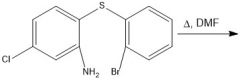
|
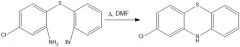
|
|
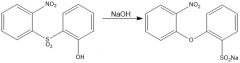
How do you smile?
|
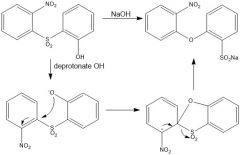
|
|
|
What is the hybridization of the triple bonded carbon in benzyne?
|
sp2
|
|
|
How do you make a benzyne?
|
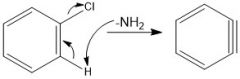
Require a super strong base
|
|
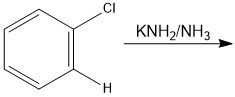
|
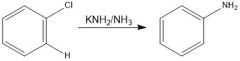
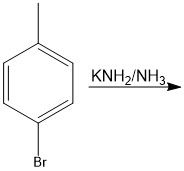
Through benzyne
|
|
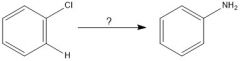
|
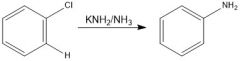
benzyne
|
|
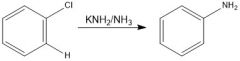
Mechanism?
|
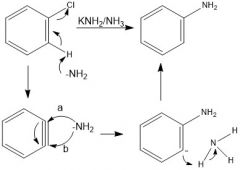
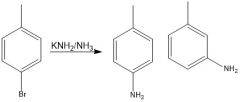
A 1:1 ratio is obtained for both paths: a & b
|
|
|
|
|
|
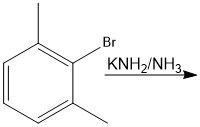
N.R.
|

