![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
37 Cards in this Set
- Front
- Back
|
Can we (mammals) store protein? |
No- there is no dedicated store of amino acids in the sense that there is no polymeric form to be a reserve of these compounds to be called upon when needed. The reserves are in the form of an amino acid 'pool' which includes free amino acids in the cells and in the blood and the rest of the reserves are in the form of functional proteins, the greatest quantity being found in muscles. |
|
|
Is dietary protein essential? |
Yes- we can only synthesise 10 amino acids ourselves and we get the other 10 by dietary means. |
|
|
Why is dietary protein essential? |
-amino acids are required as building blocks for proteins -also for synthesis of neurotransmitters, creatine, carnitine, haem, purines and pyrimidines. -they act as a source of blood glucose in fasting and starvation- (in starvation blood glucose comes from body protein) |
|
|
where is urea made? where is it excreted? |
urea is made in the liver and excreted in the kidney. |
|
|
list some key features of protein turnover... |
-Body proteins are continuously degraded to amino acids and re-synthesised. -the average turnover in an adult is 300-400g per day. -turnover is variable- most proteins have half-lives of several days but structural proteins e.g collagen may have half lives of years. -hormones and digestive enzymes are degraded very rapidly with half-lives of minutes. |
|
|
what is the amino acid pool? |
Our protein reserves are in the form of an amino acid pool. They are free amino acids present in very low concentrations inside cells or in the blood stream. They can mix and exchange with other free amino acids distributed throughout the body---> amino acid pool |
|
|
what are our protein requirements? |
-recommendation is 50-70g of protein per day. -protein is needed in the diet to replace the lost amino acids and allow for tissue repair -high protein intake is wasteful- after immediate needs are satisfied, the excess amino acids are oxidised or converted into glycogen or fat and the nitogen is excreted as urea in the urine. |
|
|
if we can't synthesise an amino acid it is classed as... |
an essential amino acid. We can't synthesise its carbon skeleton. |
|
|
non-essential amino acids are... |
synthesised in the body- we have 10 non-essential amino acids. We can synthesise their carbon skeleton. e.g glutamate, aspartate and alanine. |
|
|
pyruvate+amino group =... |
alanine |
|
|
oxaloacetate+ amino group=... |
aspartate |
|
|
a-ketoglutarate+amino group=... |
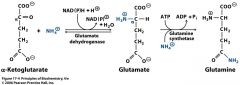
glutamate |
|
|
explain the concept of nitrogen balance... |
N intake=N excretion the total amount of nitrogen taken in the diet as protein should be equal to the amount of nitrogen excreted from the body in the form of urea, uric acid, creatine and NH4+. |
|
|
a positive nitrogen balance is... |
when nitrogen intake exceeds nitrogen excretion (protein synthesis exceeds the rate of breakdown) |
|
|
when do you see a positive nitrogen balance? |
-during normal growth in children -in convalescence after serious illness -after immobilisation after an accident -in pregnancy |
|
|
what is negative nitrogen balance... |
when nitrogen excretion exceeds nitrogen intake |
|
|
when is negative nitrogen balance seen? |
-in starvation -during serious illness -in late stages of some cancers -in injury and trauma if not corrected and is prolonged, there will be irreversible loss of essential body tissue which will ultimately lead to death. |
|
|
what are the pathways of protein degradation? |
-most cellular proteins are recognised as 'old' or damaged. They are removed by the ubiqutin breakdown system and this gives a mixture of the 20 amino acids. -foreign 'exogenous' proteins that are 'old' or damaged sub cellular proteins are taken into vesicles by endocytosis or autophagocytosis, the vesicles then fuse with the lysosomes and proteolytic enzymes degrade proteins into amino acids. |
|
|
The first steps in the breakdown of amino acids is transamination and deamination... |
Transanimation is when the amine group of the keto acid is swapped with the amine group of another keto acid- catalysed by transaminase enzymes. Deamination is the removal of an amine group from an amino acid to make ammonia. |
|
|
transanimation is useful for creating essential amino acids from non-essential ones. |
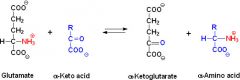
|
|
|
There is only one amino acid that can be deaminated because we only have one enzyme that can catalyse the reaction... |
glutamate is the only amino acid that can be deaminated. Other amino acids need to be converted to glutamate so they can be deaminated. Therefore in order to be deaminated, an amino acid must be converted to glutamate first. deamination is catalysed by glutamate dehydrogenase. |
|
|
Describe how amino acids (other then glutamate) are deaminated? |
1) TRANSAMINATION- transfer of the nitrogen of one amino acid to 2-oxoglutarate forming glutamate. Catalysed by amino transferase 2) the glutamate is then deaminated by the glutamate dehydrogenase. |
|
|
where does transamination take place? |
in the liver and muscle |
|
|
fate of the oxo-acids (the amino acids that have had their amino groups removed) |
after losing their amino groups, most of the 20 amino acids become keto acids they can be metabolised by the TCA cycle to C02 and H20 and provide a source of ATP in starvation, the carbon skeletons of 13 of the amino acids can also be converted back to glucose, by the liver, and these are classified as 'glucogenic' |
|
|
ketogenic amino acids... |
-Leucine and lysine can only be degraded to acetyl CoA and are therefore classified as 'ketogenic' and cannot be converted into glucose (cannot make glucose from acetyl CoA but CAN make fats from acetyl CoA) Phenylalanine and tyrosine are catabolised with part of their chemical structure being converted into glucose |
|
|
what happens to the amino group after deamination? |
The amino group enters the ornithine/urea cycle... The productuion of urea prevents the accumulation of toxic ammonia produced from amino acids. |
|
|
stage 1 of the urea cycle... |
converting ornthine back to arginine via citrulline intermediate ammonia+co2+ornithine------> citrulline Firstly, ammonia(from deamination) and co2 are converted into a reactive intermediate carbamoyl phosphate. The carbomoyl phosphate combines with ornithine |
|
|
carbamoyl phosphate molecule... |
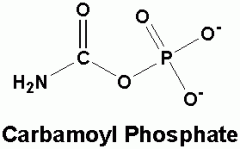
- combines with ornithine to make citrulline. |
|
|
Secondly, citrulline is converted to arginine... |
The amino group of aspartate is directly added to citrulline. |
|
|
overall diagram of the urea/ornithine cycle... |
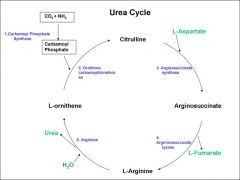
|
|
|
The liver has a central role in nitrogen metabolism... |
it is the site of... 1) removal of amino acids, glucose and fats from the portal blood supply 2)synthesis of plasma proteins (albumin, clotting factors, lipid transport proteins etc) 3)synthesis of haem, purines, pyrimidines for DNA & RNA 4)degradation of excess amino acids by transdeamination 5)conversion of NH3 to urea for excretion (urea cycle) |
|
|
The amino groups(amine) and ammonia need to be transported to the liver (so it can be converted to urea for excretion)... |
the skeletal muscle continually degrades proteins to amino acids but the liver is the only organ which can convert the amino groups of these amino acids to urea for excretion from the body The amino groups (NH2) are transported as glutamine in the blood stream |
|
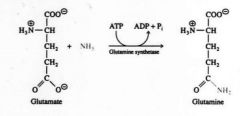
Glutamate (E) is the precursor of glutamine (Q) |
Glutamine (neutral, hydrophilic amino acid) -Free ammonia is transported in the form of the non toxic amide glutamine. Glutamine carries ammonia safely in the blood. -Glutamine can carry 2 ammonia equivalents to the liver for urea formation. -Glutamine can deliver ammonium ions to the kidney for pH regulation (buffering H+) |
|
|
list the amino acids that are important in inter-organ transport of nitrogen... |
1) Alanine (pyruvate and amino group) 2) Glutamate (oxoglutarate and amino group) 3) Glutamine (oxoglutarate and amino group) 4) aspartate (oxaloacetate and amino group) |
|
|
the end products of nitrogen metabolism are as follows... |
1) UREA- from protein breakdown 2) CREATININE- from creatine phosphate breakdown 3) URIC ACID - from DNA and RNA breakdown 4)AMMONIUM/AMMONIA - NH3/NH4+ |
|
|
Imparied conversion of NH3 to urea is known as... |
Hyperammonaemia this is due to a genetic defect which results in a reduction in catalytic activity of any enzyme of the urea cycle hyperammonaemia is a consequence of liver failure (viral hepatitis, ischaemia, liver cirrhosis) |
|
|
treatment for hyperammonaemia |
need to lower NH3 in the blood so hemodialysis, stopping protein and/or nitrogen intake and liver transplantation and/or liver cell transplantation are treatments. |

