![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
17 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Activity (A)
|
Is the quantity of Radioactive material expressed in terms of
# of radioactive atoms undergoing nuclear transformations per unit time |
|
|
|
Activity Equation
A = |
A = - dN/dt
Where dN = CHANGE in the total number of radioactive atoms (N) in a given period of time dt. *** (-) indicates the # of radioactive atoms decreases with time |
|
|
|
Activity Units
Tradiational and SI |
Tradiational = Curie
1 Ci = 3.70 x 10^10 dps SI = Bequerel 1 Bq = 1dsp |
|
|
|
1mCi = ___Bq
|
1mCi = 37 MBq
|
|
|
|
1 Ci is roughly equal to what?
|
the rate of disintegration of 1 gram of radium-226
(Ra-226) |
|
|
|
What are typical Activity values for
Nuclear Medicine Therapy with 1-131 |
Nuclear Medicine - imaging studies 0.1 to 30 mCi
I-131 Therapy - up to 300mCi |
|
|
|
Explain radioactive decay process
|
- Radioactive Decay is a random process
- Impossible to predict which radioactive atoms in a sample will decay - Over a period of time with a large sample it is possible to estimate an average rate of nuclear transformations (DECAY) |
|
|
|
Decay Constant (lamda)
(need to clarify further????) |
***has been determined by finding the average rate of nuclear transformations over a period of time with a large sample
* the Decay Constant is characteristic of each radionuclide * units in inverse time |
|
|
|
What is an isomeric transition?
Describe in what two forms in the energy released |
After radioactive decay often the daughter is still in an excited (unstable) state. Energy is released to to bring the nucleus to a lower energy state - often ground.
This decay mode is isobaric and isotonic - it occurs between two nuclear energy states and therefore their is no change in N/Z ratio Energy is released as 1) Internal Conversion Electrons and 2) Gamma rays |
|
|
|
Describe - Internal Conversion Electron
What type of transition is it part of? a) Isobaric b) Isomeric c) Isotonic |
Nuclear de-exiciation does not always result in the emission of a gamma ray. Alternatively it de-exciteds via INTERNAL CONVERSION.
Here energy is completely transferred to an orbital electron which is immediately ejected from the atom. KE = gamma E - BE The vacancy will produce and electron cascade. This is an ISOMERIC TRANSITION |
|
|
|
Describe a general decay scheme diagram
What points to the Right? What points to the Left? |
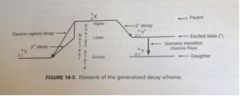
Left: electron capture (straight), Beta + decay(bent), alpha(bent)
Right: Beta - decay(straight) |
|
|
|
1 Curie = ______ dps
|
3.70 x 10^10
|
|
|
|
What decay scheme results in only a neutrino and energy?
|
ELECTRON CAPTURE
|
|
|
|
How do neutron-deficient radionuclides decay?
|
Positron Decay or Electron Capture DECAY
***Positron Emission requires an energy difference of at least 1.02 MeV between the parent and daughter or the Nuclide will decay exclusively via Electron Capture. |
|
|
|
Describe Electron Capture Decay
|
1) it is an alternative to positron decay
2) The nucleus caputres an orbital electron and converts a P to a N, then simultaneously ejects a neutrino 3) Net effect = Atomic # decreases by 1 (now a different element), the same mass #. Therefore it is ISOBARIC transition & increases the N/Z ratio 4)Now that there is a vacancy in an electron shell a casade will occur and result in |
|
|
|
Once there is a whole in an electron shell what occurs?
|
It will be filled by an e- from a higher-energy shell and the e- transition will result in the emission of characteristic x-rays an or / Auger x-rays
AUGER xrays -> |
|
|
|
Explain what would happen when the daughter T(1/2) is longer than the parent.
|
No Equilibrium - Daughter activity will build up, then the parent activity will eventually reach zero, the remaining daughter activity will decay with its own characteristic T(1/2).
|
|

