![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
173 Cards in this Set
- Front
- Back
|
Myathenia gravis
|
- autoimmune disease characterized by rapid muscle weakness during exercise, especially speech, eyelids
- believed to be due to antibodies that target nACh receptors, cross linking them and triggering endocytosis and degradation - Patients have decreased nACh receptors at the neuromuscular junction, shown by decreased EMG response - treated with AChE inhibitors, preventing ACh degradation |
|
|
Olfactory Nerve
|
- I
- Sensory - Sense of smell Test: use standard smells |
|
|
Optic Nerve
|
- II
- Sensory - Vision Test: measure acuity and integrity of visual field |
|
|
Occulomotor nerve
|
- III
- Motor - exit: superior colliculus - eye movements (superior/inferior/medial rectus, inferior oblique), pupil constriction, eyelids Test: look for the extent of visual field in each direction, ptosis, pupil dilation |
|
|
Trochlear nerve
|
- IV
- Motor - exit: inferior colliculus (posterior brainstem), dessucates before exiting, providing contralateral innervation - Eye movements (superior oblique) Test: can't look down when eye is abducted |
|
|
Trigeminal nerve
|
- V
- Sensory & Motor - exit: pons - somatic sense of the face, mouth and cornea; muscles of mastication Test: sensation on face; palpate masseter and temporal muscles |
|
|
Abducens nerve
|
- VI
- Motor - exit: medial pontomedullary junction - eye movement (lateral rectus) Test: can't look laterally, eye deviates medially |
|
|
Facial Nerve
|
- VII
- Sensory & motor - exit: pontomedullary junction - facial expression, anterior taste, lacrimal & salivary glands Test: facial expression, taste on anterior tongue |
|
|
Vestibulomotor nerve
|
- VIII
- Sensory - exit: pons - Hearing & sense of balance Test: test auditory function with a tuning fork, calorie test of vestibular function |
|
|
Glossopharyngeal Nerve
|
- IX
- Sensory & Motor - exit: rostral medulla - sensation in the pharynx; posterior taste; Test: swallowing, gag reflex |
|
|
Vagus nerve
|
- X
- Sensory & Motor - exit: rostral medulla - most parasympathetic function; Autonomic gut function; pharynx sensation; vocal cords; swallowing Test: swallowing, gag reflex, and hoarseness |
|
|
Accessory Nerve
|
- XI
- Motor - exit: rostral medulla - Shoulder & Neck Test: test sternocleidomastid and trapeziod muscles |
|
|
Hypoglossal Nerve
|
- XII
- Motor - exit: rostral medulla - Tongue movement Test: deviation of tongue during protrusion (points to side of lesion) |
|
|
General Somatic Efferent (GSE) Cranial Nerves
|
CNs: III, IV, VI, XII
- Lower motor neuron (LMN) fibers innervating somite derived structures |
|
|
Special Visceral Efferent (SVE) Cranial Nerves
|
CNs: V, VII, IX, X, XI
- Lower motor neuron (LMN) fibers innervating branchial arch derivative structures |
|
|
General Visceral Efferent (GVE) Cranial Nerves
|
CNs: III, VII, IX, X
- Pre-ganglionic bibers of the parasympathetic nervous system |
|
|
Dissociated sensory loss
|
Because the mechanosensory pathway (dorsal-medial lemniscus) ascends ipsilaterally while the pain and temperature neurons (spinothalamic) ascend contralaterally, a lesion on the spinal cord will result in sensory loss of touch, pressure, vibration, and proprioception below the lesion ipsilaterally and diminished sensation of pain contralaterally
|
|
|
Brachial motor nuclei
|
- Somatic visceral fibers
- innervate certain midline skeletal structures embryologically derived from the branchial arches - includes: muscles of facial expression, mastication, swallowing/speech in pharynx/larynx, part of the shoulder |
|
|
Trigeminal motor nucleus
|
- brachial lower motor neuron
- controls muscles of mastication |
|
|
Nucleus ambiguus
|
- brachial lower motor neuron
- controls muscles of the pharynx and larynx |
|
|
Edinger-Westphal nucleus
|
- visceral motor nucleus
- controls parasympathetic pupil contriction |
|
|
Nucleus of the solitary tract
|
- General visceral afferent fibers
- receives information for autonomic reflexes such as regulation of blood pressure by baroreceptors |
|
|
Muscle spindle
|
- sensory organs arranged in parallel with the contractile fibers of skeletal muscle.
- contains specialized receptors for detecting muscle length and changes, and the velocity of length change - stretching of the fibers causes depolarization at the ends, initiating the stretch reflex - Gamma-motor neuron efferent fibers can simulate stretch internally by contracting the interspindle fibers and triggering afferent "stretch" signaling |
|
|
Golgi tendon organ
|
- located in the tendon of muscle, which is not sensitive to stretch.
- when muscle contracts, it becomes stiff and discharges - senses/regulates muscle force and activity |
|
|
The stretch reflex
|
- responds to inputs that change muscle length and its position relative to the joint
- stretching stimulates the spindle which triggers the alpha MN's to contract the muscle, contraction triggers the golgi tendon organ which modulates contraction - reflex compensation is aided by reciprocal inhibition of antagonist muscles and voluntary control of alpha and gamma motor neurons - reflex cannot fully compensate for stretch unless voluntary input is involved |
|
|
Reticulospinal pathway
|
- medial upper motor neuron pathway that coordinates trunk and proximal limb movement
- drives flexors in lower limb and inhibits extensors, opposite pattern in upper limb. (balanced in reflexes by the vestibulospinal tract resulting in resting posture) - originates in the pontine and medullary reticular formation, descends bilaterally (medially) to the spinal cord - driven by input from cerebral upper motor neurons |
|
|
Vestibulospinal motor pathway
|
- medial upper motor neuron pathway that coordinates trunk and proximal limb movement
- drives extensors in lower limb and inhibits flexors, opposite pattern in upper limb. (balanced in reflexes by the reticulospinal pathway resulting in resting posture) - originates in the lateral vestibular nucleus, descends bilaterally (medially) to the spinal cord - driven by autonic input from the vestibular organs of the ears |
|
|
Signs/symptoms of upper motor neuron damage
|
- weakness (lateral or medial pathways)
- spasticity: increased tone, hyperactive deep reflexes, clonus - Babinski's sign (lateral tracts) - loss of fine voluntary movements (lateral tracts) - loss of coordinated trunk movement (medial tracts) |
|
|
Cranial Hematomas
|
= cranial bleeds associated w/ increased intracranial pressure
Epidural (between dura and skull) - acute presentation: lucid interval, delayed neurological signs - high pressure arterial bleed (middle meningeal) so accumulates rapidly - often follows direct blow to the skull (+/- fracture) Subdural (between dura and arachnoid) - delayed presentation - low pressure venous bleed (bridging veins) so accumulates more slowly - often follows more serious brain injury where brain is shaken inside skull (car wreck, fall) |
|
|
Brain herniations
|
= secondary injury in response to increased intracranial pressure (brain swelling, bleeds)
Tonsilar: - cerebellar tonsil pushed into the brainstem/through foramen magnum - pressure on the medulla oblongata (respiratory center) may lead to respiratory arrest Uncal/Tentorial: (usually precedes tonsilar) - uncus of the temporal lobe herniates through tentorium cerebelli - pressure on CN3 impairs pupillary light reflex (lose contraction). |
|
|
General functions of the cerebral lobes
|
Frontal: conscious thought; damage results in mood changes, social differences, etc
Parietal: integration of sensory information and manipulation of objects (portions involved w/ visuospacial processing) Occipital: sensation of light/sight; damage can produce hallucinations Temporal: sense of smell and sound, processing of complex stimuli like faces and senses Insular: autonomic and visceral systems (pain, taste, equilibrium) |
|
|
Primary sensory cortexes
|
- primary somatosensory: post central gyrus, receives sensory information (touch, proprioception, pain, temp) from the spinal cord via the thalamus. Somatotopic organization, contralateral representation. Sensory association complex (recognition/meaning for same type of sense, ex: 5 fingers) and multimodal association complex (multiple senses, memory) refine and integrate information, which then is used to stimulate the premotor and primary motor cortexes
Primary gustatory: near insular cortex (on roof of lateral sulcus near base precentral gyrus) Primary visual: narrow arrow on the medial and tip of the occipital lobe (calcarine sulcus). Surrounding association areas process color, shape, and movement. Complex processing extends to the temporal and parietal lobe. Left occipital lobe processes the right visual field. Primary auditory: superior temporal lobe along the lateral sulcus. Recognizes characteristics of sound (pitch, volume, rhythm). Association complex is posterior. On left (usually) is the Wernicke’s center which overlaps w/ the auditory association complex (includes receptive language center). Corresponding right side center often used to understand tonality of speech or affect. Anterior association area: in prefrontal cortex, receives info from posterior association areas, integrates past experience, initiates/plans motor movements, linked to limbic system. Performs complex functions: thinking, perceiving, intentionally remembering, processing abstract ideas, reasoning, judgment, impulse control, mental flexibility, social skills, humor, empathy, conscience |
|
|
Primary Motor Cortexes
|
Primary motor: precentral gyrus (containing upper motor neurons/pyramidal cells that control muscles in specific areas of the body--homunculus)
Premotor cortex: anterior precentral gyrus, planning area that send axons to the primary motor cortex to coordinate muscle stimulation (controls voluntary actions dependent on sensory feedback) Frontal eye field: anterior to premotor cortex, coordinates voluntary eye movement (premotor) Broca’s area: left hemisphere (anterior and low to premotor), premotor area for speech (manages speech production, connected to Wernicke’s/language) |
|
|
Damage to medial brainstem
|
- contralateral weakness: loss of corticospinal tracts which decussate in the medulla
- ipsilateral cranial motor deficits: all CNs except 4 innervate ipsilaterally - contralateral loss of fine touch: damage to dorsal column/medial lemniscus pathway which decussate in the caudal medulla (gracile/cuneate nuclei) |
|
|
Damage to lateral brainstem
|
- contralateral loss of pain/temp sensation: damage to spinothalamic tracts which decussate immediately at the spinal level
- ipsilateral loss of facial sensation: damage to trigeminal nuclei/tract - nystagmus: damage to vestibular nuclei - ataxia: damage to cerebellar peduncles - Horner’s syndrome (ptosis + miosis +/- ipsilateral anhydrosis): due to descending sympathetic autonomic tract damage |
|
|
Seizures
|
= abnormal synchronous discharge of cortical neurons resulting in paroxysmal change in behavior, sensation or motor activity
- focal or generalized onset, provoked or unprovoked - occurs in 9% of the population (often kids when febrile) Epilepsy = two or more unprovoked seizures - 1-2% of population Dx: history/exam, EEG, MRI, video-EEG monitoring |
|
|
Non-epileptic causes of seizure
|
Physiologic:
- syncope, TIA, migraine, sleep disorder, movement disorder Psychiatric: - conversion, panic attack, ADHD, breath-holding |
|
|
Generalized Tonic-clonic seizure
|
= Grand mal
Signs: - loss of consciousness - duration 1-2 min - fall with muscular rigidity phase (tonic) followed by rhythmic jerking (clonic) phase - inhibited respiration (cyanosis), ictal cry at start (air forced out of lung w/ diaphragm contraction) - lateral tongue bite - bladder/bowel incontinence - postictal confusion |
|
|
Absence seizure
|
= petit mal
Signs: - brief loss of awareness, “staring spell” - duration 5-20 seconds - subtle movements: eye blink, head nod - no postictal period |
|
|
Myoclonic seizure
|
Signs:
- brief, shock-like muscle contractions - usually involves head, shoulders, upper extremities - consciousness is preserved - precipitated by waking - may progress to GTC seizure |
|
|
Atonic seizure
|
= “drop-attacks”
Signs: - sudden loss of consciousness - duration of a few seconds - frequently associated w/ falls, injuries are common (dangerous). Helmet recommended - tend to be very frequent (many/day), very hard to treat - almost always connected to developmental delay |
|
|
Epilepsy syndromes
|
Idiopathic/Genetic: (exam and MRI are normal)
- no underlying pathology - normal development - relatively self-limited - medication-responsive - genetic predisposition - generalized > focal Symptomatic/Structural/Metabolic: - definite neurological abnormalities - identifiable underlying cause - frequent seizures - difficult to control - focal or generalized, but focal > generalized |
|
|
Childhood absence epilepsy
|
Class: idiopathic generalized epilepsy
Seizure Type: absence Clinical: - onset age 3-8, usually resolves by teens - frequent daily seizures w/ 3Hz wave Good prognosis |
|
|
Juvenile Myoclonic Epilepsy
|
Class: idiopathic generalized epilepsy
Seizure Type: myoclonic, GTC Clinical: - onset 13-20y, normal development - Rare GTC, myoclonic seizures in the morning - 4-5 Hz spike wave Prognosis: requires lifelong treatment |
|
|
Lennox-Gastaut Syndrome
|
Class: symptomatic generalized epilepsy
Seizure type: Any (atonic, GTC, tonic) Clinical: - age of onset 1-6y - a/w mental retardation, abnormal MRI - very frequent seizures - 1-2 Hz slow spike wave Prognosis: poor, very difficult to control often requiring several medications |
|
|
Benign Epilepsy w/ Centro-temporal Spikes (BECTS)
|
Class: idiopathic partial epilepsy
Seizure type: simple partial, secondary GTC Clinical: - onset 4-10y, normal development - infrequent nocturnal seizures - centro-temporal spikes Prognosis: good, remission by teens |
|
|
Temporal lobe epilepsy
|
Class: symptomatic partial epilepsy
Seizure type: simple partial, complex partial, occasional GTC Clinical: - onset at any age - a/w mesial temporal sclerosis (scaring down of hippocampus) - may or may not have cognitive dysfunction (esp. memory loss) - temporal spikes on EEG Prognosis: poor for remission, often refractory to meds but can have good outcome w/ surgery |
|
|
Epilepsy
|
= two or more unprovoked strokes
- 1-2% of population, multiple etiologies Risk factors: - penetrating head wounds (subdural hemorrhage), severe head trauma and stroke, severe neonatal hypoxia, genetic contribution Management: - 70% anticonvulsants, 20% surgery, 10% intractable |
|
|
Carbamazepine
|
= Tegratol
- anticonvulsant (partial and GTC) Mechanism: stabilizes inactivated voltage-gated sodium channels, making neurons less excitable until the drug dissociates - P450 inducer: T1/2 shortens from 36h to 12h with chronic treatment - also used for neuropathic pain, bipolar disorder Adverse reactions: diplopia, drowsiness, ataxia, potentially suicidal behavior or ideation , teratogenic (take excess folate to minimize) |
|
|
Phenytoin
|
= dilatin
- anticonvulsant Mechanism: stabilizes inactivated voltage-gated sodium channels, making neurons less excitable until the drug dissociates - highly bound to plasma protein, limited capacity for metabolism (1+2 saturation kinetics) Adverse effects: dizziness, drowsiness, gingival hyperplasia, hirsutism, coarsening of facial features |
|
|
Lamotrigine
|
= Lamictal
- anticonvulsant - indications: focal seizures, also primary/secondary GTC, Lennox-Gastaut syndrome. Also mood stabilizer (bipolar) Mechanism: stabilizes inactivated voltage-gated sodium channels, making neurons less excitable until the drug dissociates Adverse reactions: skin (steven’s-johnson, DRESS, toxic epidermal necrolysis), interaction w/ OCPs, visual disturbance, balance/coordination disturbance |
|
|
Ethosuximide
|
= Zarontin
- anticonvulsant: first line for absence epilepsy Mechanism: inhibits T-type Ca channels on thalamo-cortial relay neurons (not involved in transmitter release0 - no plasma protein binding, T1/2 of 40-60h, renal excretion Adverse effects: weight loss, nausea, diarrhea |
|
|
Valproate
|
- anticonvulsant (2nd most commonly used worldwide, most common in US): all partial and generalized seizures (incl. absence, GTC, myoclonic). Also for bipolar, migraine
Mechanism: inhibits Na ion channels, inhibits GABA deactivation via GABA transaminase (may also stimulate GABA synthesis) resulting increased GABAergic inhibition Adverse effects: PCOS, weight gain, hepatotoxicity (esp. in infants 1/500), teratogenic (take excess folate to minimize) |
|
|
Lorazepam
|
= Ativan
- benzodiazepine anticonvulsant: for status epilepticus, acute seizure relief Mechanism: binds to receptor on GABA-A receptors in the CNS to enhance GABAergic inhibition Adverse effects: sedation, hypotension, ataxia, amnesia |
|
|
Status Epilepticus
|
= seizure or seizure cluster lasting >30min
Causes: anticonvulsant non-compliance, withdrawal from ETOH or barbiturates Complications: cerebral injury, mortality 35% at 30d in adults if SE>60min Tx: (goal flatline EEG) clear airway, check glucose/electrolytes. Initial: lorazepam/diazepam, then phosphenytoin, phenobarbital, propafo |
|
|
Vagal nerve stimulator
|
= alternate therapy for epilepsy, mechanism unknown
- intermittent programmed electrical stimulation of the vagus nerve (30sec on, 5min off) - option for patient triggered stimulation (auras) Adverse effects (related to stimulus): hoarseness, throat discomfort, dyspnea |
|
|
Med choice for seizure type
|
Partial complex: carbamazepine, phenytoin, gabapentin, vigabatrin, topiramate, tiagabine, levetiracetam
GTC: phenytoin, valproate, carbamazepine Absence: ethosuximide, valproate (if TC also present) Myoclonic: valproate, clonazepam |
|
|
Signs of Left (dominant) hemisphere lesion
|
Right visual field defect
Right hemiparesis Right Hemisensory loss Left gaze deviation (left gaze preference): losing gaze center that moves eyes to the right Aphasia (language center usually on dominant side) |
|
|
Signs of Right (non-dominant) hemisphere lesion
|
Left visual field defect
Left hemiparesis Left hemisensory loss Left hemineglect Right gaze deviation |
|
|
Localization of brain lesion by symptoms
|
Lethargy/delirium Bicerebral hemispheres
Seizures Cerebral cortex Memory Thalamus or medial temporal lobes Hemibody sensory/motor Contralateral hemisphere Visual field Contralateral, posterior optic chiasm Language Left cerebral cortex Neglect Right cerebral cortex |
|
|
Signs of brainstem lesion
|
Quadriparesis and bilateral sensory loss OR crossed deficits (face vs. body)
↓ Consciousness (reticular activating system) Nausea vomiting (area postern) Hiccups and respiratory depression (medulla) Eye movement abnormalities: diplopia (3,4,6), gaze palsies Vertigo and Tinnitus (CN VIII) Dysarthria and dysphagia: tongue (CN XIII), oropharyngeal (CN IX, X), facial weakness (CNVII) |
|
|
Signs of cerebellar lesion
|
- generally loss of motor coordination
Ipsilateral limb ataxia (appenidicular) Truncal or gait ataxia (midline) |
|
|
Spinal cord lesion
|
Signs Below lesion:
- weakness, numbness (“tight belt) at level of lesion, “hung level” ↓sensation a few dermatomes below) - hyperreflexia, hypertonia - autonomic dysfunction: urinary incontinence, constipation, sexual dysfunction Causes: - transverse myelitis: MS, Devic’s syndrome, sarcoid, SLE, infection - compressive myelopathy: metastatic cancer, meningioma, central disk herniation, vertebral fracture, epidural hematoma, epidural abscess, Pott’s disease (TB)v |
|
|
Stroke risk factors
|
Non-modifiable:
- Age (↑w/ age), sex (M>F), race (AA), geographic region (southern US, NW US) Modifiable - HTN (systolic BP, anti HTN therapy), DM, smoking, CAD (cholesterol), Afib, LVH, waist-hip ratio/BMI, ETOH |
|
|
Stroke types
|
Ischemic (85%)
- clot occludes artery, brain deprive of O2, glucose required for energy production - Subtypes (TOAST criteria): 20% atherosclerotic CV dz, 30% cryptogenic, 30% lacunar (small vessel), 20% cardioembolic - mechanisms: coagulopathies, small vessel-lacunar (lipohyalinosis/atherosclerosis, embolism, vasculitic), large vessel intra/extracranial (atherosclerosis, non/inflammatory arteriopathies, Aortic arch disease), cardioembolism Intercerebral Hemorrhagic (10%) - bleeding into brain, mass effect damage, vomiting - causes: HTN, AVM, malformations, aneurysm, vasculitis, thrombosis, tumor, amyloid, hemorrhagic conversion of infarct Subarachnoid Hemorrhage (5%) - bleeding around the brain: ↑ intracranial pressure, hydrocephalus, vasospasm. TERRIBLE headache - causes: aneurysm, AVM, idiopathic, venous thrombosis, trauma |
|
|
Stroke symptoms
|
5 SUDDENs:
- weakness/numbness usually one side - difficultly speaking or understanding speech - difficultly walking, dizziness, loss of balance - loss of vision in one or both eyes - severe headache Act FAST - face, arm, speech, time |
|
|
Left MCA stroke syndrome
|
Loss of Wernicke’s/Broca’s areas (L dominant): Aphasia (loss of language production/comprehension)- fluency, repetition, naming, comprehension, reading/writing
Loss of L frontal eye field: left gaze deviation Loss of L optic radiations: right homonymous hemianopsia Loss of L motor and sensory cortex: right face/arm>leg weakness and numbness |
|
|
Right MCA stroke syndrome
|
Contralateral neglect: denial of weakness/impairment, denial of limb ownership, visual/auditory/sensory deficit
Right gaze deviation (loss of left frontal eye field) Left homonymous hemianopsia (loss of right optic radiations) Left face/arm>leg weakness and numbness (loss of R motor/sensory cortex) |
|
|
Left ACA stroke syndrome
|
+/- aphasia if hits language centers (L dominant)
Apathy (loss of frontal cortex) +/- right arm weakness Right leg weakness |
|
|
Right ACA
|
Apathy (frontal cortex)
+/- left arm weakness Left leg weakness |
|
|
Signs of Cortical vs. Subcortical lesions
|
Cortical:
- aphasia, hemineglect, astereognosis (inability to recognize by touch), hemianopsia - face/arm > leg weakness (MCA), leg > face/arm weakness (ACA) Subcortical: - absence of cortical findings - face, arm, leg equally weak w/p other deficit |
|
|
Signs of vertebral artery/posterior inferior cerebellar stroke syndrome
|
- vertigo, nausea/vomiting
- diplopia, nystagmus - hoarse voice - ipsilateral facial numbness w/ contralateral arm/leg numbness (crossed-signs) - ipsilateral ataxia - Horner’s syndrome (ptosis, miosis, anhydrosis due to disruption of sympathetic stimulation) |
|
|
Signs of Basilar artery stroke
|
- quadriplegia or hemiplegia
- locked-in syndrome - nystagmus - crossed-signs: right facial weakness, left hemiplegia - vertigo, diplopia, nausea/vomiting - ataxia |
|
|
Signs of lacunar stroke syndromes
|
= small (<1.5cm diameter), deep infarcts in the territory of deep penetrating arteries
- pure motor hemiparesis: face/arm/leg on one side w/o sensory, language, or visual abnormalities. Typically in internal capsule or pons - pure sensory stroke: isolated numbness over face/arm/leg on side w/o motor, visual or language abnormalities. Typically posterior ventral nuclei of thalamus - sensorimotor stroke: combination sensory/motor deficit on one side w/o vision or language abnormalities. Typically thalamic-internal capsule (posterior limb) junction - ataxic hemiparesis: weakness/incoordination on one side w/o other abnormalities. Typically internal capsule or pons - clumsy-hand dysarthria: contralateral facial weakness causing dysarthria plus hand weakness/incoordination. Typically internal capsule or pons |
|
|
Stroke work up
|
Dx:
Stat labs: CBC, chemistries (esp. glucose), PT/INR, PTT, cardiac biomarkers (CK, troponin), Tests: 12-lead EKG (look for Afib), non-contrast CT brain scan, cerebral angiogram (for mechanical embolectomy) or MR angiopathy or carotid duplex for origin of clot Tx: (goal: save penumbra from hypoperfusion damage) - If acute ischemia (< 3hrs) give IV t-PA, but NOT if subacute (if blood seen on CT do not give) - do not lower BP (except in AMI, ARF, Ao dissection, HTN encephalopathy, IV t-PA candidate): acutely will rise to maintain perfusion to penumbra - intra-arterial t-PA (not FDA approved) w/in 6hrs - Merci retriever: surgical removal of embolus - Aspirin: r/o hemorrhagic first, improves M&M w/in 48hrs |
|
|
Tremor
|
= involuntary rhythmic oscillating movement that results from alternating or synchronous contraction of reciprocally innervated antagonist muscles
Types: - rest: occur in repose, while resting limb - postural: while body part is maintain posture against gravity - kinetic: occurs during goal directed movements - mixed: any combination of the above - action: postural or kinetic - intention: action or kinetic - task specific: occurs w/ specific tasks: writing, instrument playing Causes: essential tremor, physiologic tremor, parkinson’s disease |
|
|
Rigidity
|
= increased resistance to passive movement, velocity-independent
Types: “lead-pipe,” “cogwheel” - patients may complain of stiffness but not a major source of disability |
|
|
Akinesia/Bradykinesia
|
= slowness, fatiguing, arrests in on-going movement
- decreased amplitude of movement - interferes w/ all activities, one of the most disabling features of parkinsonism. Results in many additional features and complaints |
|
|
Dystonia
|
= involuntary sustain muscle contractions producing twisting or squeezing movements and abnormal postures
- may have stereotyped, repetitive movement that vary in speed from rapid to slow and that may result in fixed postures from sustain contraction - may be associated with tremor - may be relieved by positing, specific activities |
|
|
Chorea
|
= a state of excessive, spontaneous movements irregularly timed, non-repetitive, randomly distributed, and often w/ dance-like quality that involves multiple body parts
Causes: Huntington’s disease, drug induced (Tardive dyskinesia) |
|
|
Tic
|
= repetitive, brief, rapid, involuntary, purposeless, stereotyped movements that involve single or multiple groups
- can be a patterned sequence of coordinated movements that may be complex or simple |
|
|
Myoclonus
|
= rapid, shock-like, arrhythmic (usually), and often repetitive movements
- may be focal, multifocal, or generalized - negative myoclonus = sudden loss of tone, asterixis Causes: metabolic disturbances, structural brain damage, epilepsy |
|
|
Parkinson’s Disease Motor Features
|
Cardinal:
- Resting tremor (often starts unilateral) - Muscle rigidity w/ cogwheeling - Bradykinesia: slowing of ADLs - Akinesia: masked face, freezing, difficulty arising from chair, decreased arm swing, difficulty turning in bed Other important ones: - Gait disorder w/ postural change: festination, postural instability, stooped posture, shuffling gate, freezing, loss of arm swing - hypophonic speech - sialorrhea - anosmia - dystonia, pain - autonomic dysfunction: GI disturbances (constipation), orthostatic hypotension - sleep disturbances: REM behavior disorder - depression (40-50%) - cognitive dysfunction: executive function losses - may overlap w/ essential tremor, restless leg syndrome |
|
|
Basal Ganglia circuitry
|
- interacts w/ the cortex via the thalamus w/ separate circuits for motor, executive and limbic functions
Input from cortex (stimulatory/glutamatergic) to the striatum and subthalamic nucleus Output (inhibitory/GABAergic) to the cortex (via thalamus) from internal globus pallidus and substantia nigra pars reticulata In PD: basal ganglia output increased, basal ganglia and thalamus engaged in abnormal oscillatory bursting activities which disrupt cortical operations |
|
|
Parkinsonism vs Parkinson Disease
|
PD = bradykinesia plus 1 of 4: muscle rigidity, 4-6hz resting tremor, postural instability
Parkinsonism = symptoms associated w/ PD but due to other disease/process (neurodegenerative or hereditary disorders, toxins/drugs, infections, structural abnormalities) Onset: Asymmetric typical for PD, symmetric typical of drug induced parkinsonism (also neurodegenerative disorders) Tremor: typical for PD Progression: PD progresses smoothly, vascular parkinsonism is stepwise Other symptoms (unlikely w/ PD): cerebellar signs, early autonomic failure, gaze palsy, early dementia |
|
|
Parkinson’s disease epidemiology
|
Incidence: 5-24/million worldwide (USA 20.5/mil), rising with the aging of population
Prevalence: USA/Canada: 300/mil, total 1 million patients, Age-dependent increase Onset: mean 62.4 years. Rare before 30; 4-10% cases before age 40 (considered genetic, vs older are sporadic) - Male > Female Risks: definite: age, MPTP toxin. Possible: toxins (herb/pesticides, heavy metals, wood preservatives, proximity to industry, rural residence, well water use), head trauma. Possible protective: smoking, caffeine, anti-inflammatory meds Genetics: <20% monogenetic (PARK gene mutations: young, rapidly progressive, prominent dystonia), susceptibility loci identified in sporadic/late onset cases (ex: glucocerebrosidase mutations) Severity: severity of dopamine loss correlates w/ parkinsonism but not w/ balance problems, dementia, autonomic insufficiency. ~70%: first symptoms, >90% loss: advanced symptoms |
|
|
Parkinson’s Disease Pathology
|
- pallor of the substantia nigra due to degeneration of the dopaminergic cells
- Lewy bodies: cytoplasmic inclusions in neurons of the substantia nigra (stain pink on H&E). Composed of α-synuclein filaments (staining may show along axon – lewy neurite) - loss of spines on neurons in the striatum due to dopamine deficiency L-DOPA PET scan: progressive loss of dopamine from the striatum esp. the putamen Location: may ascend brainstem to cortex, pathology also outside brain in spinal cord, CV innervation (sympathetic deinnervation→ orthostatic hypotension), GI tract (aspiration) |
|
|
Mechanisms for dopaminergic degeneration in Parkinson’s disease
|
- oxidative damage: the cells are very sensitive to oxidation, results in apoptosis
- mitochondrial damage: resulting in altered function from enzyme loss - inflammation: some anti-inflammatory meds actually prevent PD to some extent - defective proteasome function due to loss of ubiquitin ligase parkin - protein aggregation (α-synuclein): may interfere w/ microtubule function, may promote tau protein aggregation |
|
|
Treatment for Parkinson’s disease
|
Neuroprotection: none proven, aerobic exercise probably good (remember safety given movement disorder)
Symptomatic relief: - no functional impairment: do not treat - mild symptoms (tremor, bradykinesia, rigidity, dyskinesias): amantadine (mildly affective against all symptoms), selegiline (MAO-B inhibitor), COMT inhibitors - discrete symptoms: tremor/rigidity (anticholinergics, but LOTS of neural side effects), depression (antidepressants), anxiety (benzodiazepines) - severe symptoms: dopamine agonists (can be taken w/ protein, longer T1/2, mono/adjunct therapy. Side effects: psychosis, hallucinations, N/V motor fluctuations), L-DOPA/carbidopa +/- entacapone (L-dopa is neutral, needs transporter to cross BBB, inhibit peripheral conversion to DA and N/V side effects. Efficacy can wear off, or On/Off freezing effects) - Refractory to medical therapy: deep brain stimulation (high frequency electrical stimulation) |
|
|
Dystonia
|
= slow, sustained involuntary muscular contractions and abnormal postures
- co-contraction of agonists and antagonists: disturbed temporal sequences of in/activation - symptom, rather than a disease, but have consistent appearance - Contractions often brought on by voluntary movement (“action dystonia”), worsen w/ stress and fatigue. May be lessened w/ sensory tricks - Contractions may progress to other body regions leading to abnormal postures Classification: early vs late onset (</> 21y), focal/segmental/multifocal (more common, action dependent) vs generalized (more genetic, childhood—DYT1), primary vs secondary |
|
|
Primary torsion dystonia (DYT1)
|
= Generalized, progressive dystonia of childhood
Genetics: AD w/ 30% penetrance, GAG deletion of DYT1 locus (Chr 9) which codes for protein torsinA (physiologic effect of mutation unclear) Epi: mostly Ashkenazi Jews, early onset (<21y) Clinical: usually begins in one limb (leg) and spreads to the rest of the body (limbs, trunk) Genetic testing: recommended for primary dystonic <21y, positive FHx helpful (neg. is not). May skip b/c results have no impact on treatment, not good for screening. |
|
|
Focal dystonia
|
= most common type of dystonia in adult patients
- usually action dependent Types: cervical dystonias (troto/latero/antero/retrocollis: head turning), blepharospasm (eyelid spasm causing blindness b/c can’t open eyes), overuse dystonia (writer’s cramp) - Pathophys is unclear |
|
|
Workup for dystonia
|
R/o: Wilson’s disease (serum ceruloplasmin , 24hr urine Cu excretion, LFTs, slit lamp exam), Dopa-responsive dystonia (L-DOPA trial)
Rx: anticholinergics (require large dose often not tolerated in adults, mechanism unclear), muscle relaxants (benzodiazepine, baclofen: not very effective) Sx: pallidotomy (not done anymore), deep brain stimulation of the globus pallidus (generalized dystonia, cervical/treatment refractory secondary dystonias. Slow onset of effect) Botulinum toxin (for focal dystonia: cervical, bleopharospasm, laryngeal, limb): blocks local ACh release at neuromuscular junction→ partial muscle paralysis (lasts 3mo). S/E antibodies causing resistance, excessive muscle weakness PT: minimal effect, braces may help provide sensory tricks |
|
|
Tremor classification
|
Kinetic:
- physiologic tremor - essential tremor (occurs w/ movement, absent at rest) - task or position specific tremor: writing, voice - metabolic disturbance: hepatic encephalopathy (asterixis), hypoglycemia, hyperthyroidism, hyperparathyroidism, hypocalcemia - drug induced: valproic acid, phenytoin, bronchodilators (beta agonists), lithium, cyclosporine, SSRIs, caffeine - peripheral neuropathies - dystonia Tremor at rest: - Parkinsonism, Parkinson’s Disease |
|
|
Essential tremor
|
= kinetic tremor that is present throughout movement but absent at rest (postural and/or kinetic)
- 4-12Hz, amplitude can increase over time - exaggerated by stress, fatigue, caffeine, diminished by alcohol Location (often asymmetric): 90% upper extremity, 50% head, 30% voice, 15% leg or chin Epi: most common movement disorder of adults (1-6% of pop), bimodal onset at 20s’ and 60’s, often familial, M=F, rapid progression in younger and those w/o head tremor. No clear effect on mortality Genetics: AD w/ variable penetrance, 50% have 1st degree relative, 5x increase for relatives. No known non-genetic risk factors Pathogenesis: unknown, possibly abnormal central oscillator involving brainstem or disturbance of GABAergic transmission Tx: beta-blockers (propranolol), primidone (barbiturate precursor), benzodiazepine (GABA agonist), botox, surgery (thalamootomy or thalamic deep brain stimulation-universally responsive) |
|
|
Tardive dyskinesia
|
= involuntary movements (other than tremor) resulting from treatment with a neuroleptic drug for >3mo (or 1mo if >60yo)
Causative agents: phenothiazines, thioxanthines, butyrophenones (haloperidol), dibenzoxazepine (loxapine), others (metoclopramide, amoxapine, flunarizine) Epi: mostly elderly patients (50-75%, 20% year on these drugs) Risk factors: more severe in women >50, structural brain damage (epilepsy, dementia, ECT), DM, substance abuse, negative symptoms of schizophrenia, african american Tx: discontinue/reduce antipsychotics if possible (may result in worsening of symptoms), discontinue anticholinergics, adjuvant therapy, surgery (target lesion or GPi DBS) Prognosis: 50% reversible (80% in younger patients, 25-50% in those on continued antipsychotics), remission in 5y of drug withdrawal. Tardive dystonia has poor prognosis |
|
|
Ataxia
|
= clinical syndrome of incoordination (abnormal regulation of movement speed, force, distance)
Manifestations: - Motor: movement delay (esp. deceleration), dysmetria, dysrhythmia, dyssynergia, dysdiadochokinesia (irregular alternating movements), bradykinesia, hypotonia, postural limb tremor - eye: gaze-evoked nystagmus, impairment in smooth pursuit and saccades, inability to suppress the vestibule-ocular reflex by fixation Pathogenesis (proposed): hyper-excitability of deep cerebellar nuclei |
|
|
Ataxia classification
|
Acquired:
- Primary: idiopathic late-onset cerebellar degeneration, Multiple Systems Atrophy, autoimmune (paraneoplastic cerebellar degeneration: anti-Purkinje Cell Abs, anti-GAD65, gluten ataxia, others—thyroid, etc) - Secondary: hypothyroidism, stroke, multiple sclerosis, tumor, vitamin E deficiency, toxic (alcohol, lithium, antiepileptic) Inherited - Primary: AD (spinocerebellar ataxia), AR (Friedreich’s Ataxia, Baltic myoclonus-epilepsy w/ ataxia; others), X-linked (sideroblastic anemia, fragile X tremor syndrome), mitochondrial (neuropathy and ataxia w/ retinitis pigmentosa (NARP)) - Secondary: inborn errors of metabolism, |
|
|
Ataxia workup
|
PMH, PE, FHx :look for evidence of genetic inheritance, social triggers, character of ataxia
Tests: head MRI, labs (TSH, B12, Vit E, anti-GAD, anti-gliadin, anti-TTG, ceruloplasmin/copper, anti-thyroperoxidase, paraneoplastic Abs, inflammatory markers), genetic panel if affordable Tx: Symptomatic for ataxia: PT for balance, rx (riluzole, Chantix for FXTA) Symptomatic for secondary symptoms: dysphagia, spasticity, pain, depression Disease modifying treatment (not really available): lithium for SCA1, dantrolene for SCA2, HDAC inhibitors + antioxidants for FRDA |
|
|
Friedrich’s Ataxia
|
= most common inherited ataxia
- Genetics: AR, 1/60-90 are carriers, 1/30K incidence, strong founders effect, childhood/adolescent manifestation Pathogenesis: expansion of GAA repeats in intron 1 of nuclear encoded mitochondrial protein frataxin. Normal 7-36, disease 100-2000: cause abnormal DNA structures, abn ox-phos, increased susceptibility to oxidative stress, reduction of Fe-S cluster protein synthesis, mitochondrial iron accumulation Systems affected: spinal cord (dorsal columns, spinocerebellar tracts, corticospinal tracts), PNS (dorsal root ganglia, sensorimotor loss), CNS (brainstem, cerebellar nuclei), heart (fibrotic cardiomyopathy, Fe deposits) Clinical: - neurological: ataxia/gait instability, dysarthria, areflexia, Babinski sign, loss of vibrational/positional sense - non-neurologic: cardiomyopathy, glucose intolerance or DM, scoliosis, pes cavus (high foot arch) Tx: - supportive: balance training, orthotics - antioxidants: minimal success |
|
|
Anti-GAD ataxia
|
= ataxia due to autoimmunity against GAD65 (produces GABA in brain and pancreas) and other GAD proteins
Clinical: diabetes, movement abnormalities (downbeat nystagmus, ataxia), epilepsy, autoimmune encephalitis, stiff person syndrome (w/ high Abs). Symptoms may be chronic or acute and vary from pure cerebellar to SPS/brainstem/limbic Treatment: - symptomatic: 4-aminopyridine for downbeat nystagmus, diazepam for stiffness - immunosuppression: IVIg, steroids - rehabilitation: PT, walker |
|
|
Huntington’s Disease
|
= slowly progressive, degenerative neuropsychiatric disorder
Epi: 5-10/100K, 35K in the US, 90% adult onset, 10% juvenile (post-reproductive age), duration 15-30+ years Genetics: AD w/ high penetrance, CAG repeats in huntingtin protein. Normal <26, mutable 27-35, intermediate/incomplete penetrance 36-39, disease >=40 (very long repeats →juvenile onset) Pathogenesis (proposed): gain of function mutation disrupts cellular/neuronal metabolism causing toxicity. N-terminus and overall shape contribute to toxicity. Expressed universally but effects confined to the brain Cardinal signs: movement disorder, emotional disturbances, cognitive decline Micro: loss of medium spiny nerve projections, inclusions of huntingtin protein in cell bodies of striatal nerves Macro: variable degeneration of neuronal cells (high in striatum→ severe caudate atrophy, basal ganglia dysfunction), expansion of CSF on MRI, reduced brain mass (20%) |
|
|
Huntington Disease Clinical presentation
|
1. FHx w/ typical motor sight or genetic confirmation
2. presence of choretic movement disorder 3. No other explanation for symptoms Other key features: Cognitive decline: - ↓intellectual speed and flexibility : slowed thinking, distractability - ↓ visual special abilities: reduced awareness to environment and body in space, may get lost - ↓ memory: ↓ efficiency of learning new info, ↓ speed of retrieval of old info (all still there , just longer to access) - ↓ executive function: trouble w/ organization, regulation, awareness Emotional Disturbance: depression, impulsivity, irritability, OC behavior, anxiety, psychosis, personality or behavioral changes Movement disorder: eye movement abns, chorea, motor impersistence, dystonia and bradykinesia, less common (tics, tremor, myoclonus) Juvenile onset: rigidity, tremor, seizures, dystonia, myoclonic jerks, school failure, behavioral problems |
|
|
Huntington Disease workup
|
Dx:
1. FHx w/ typical motor sight or genetic confirmation 2. presence of choretic movement disorder 3. No other explanation for symptoms DDx: tardive dyskinesia (r/o hx of narcoleptic use), DRPLA ataxia (genetic testing), thyrotoxicosis (Thyroid function studies), SLE (vasculitis, do ANA), cerebrovascular disease (MRI, risk factors), Wilson’s disease (ceruloplasmin/copper, slit lamp exam), neuroacantocytosis (spear, CPK, conduction studies) Genetic Testing: for symptomatic patients (confirmatory), unaffected at-risk adults (predictive), at risk fetuses (prenatal/preimplantation testing), unaffect children at risk (not done) Tx: none available to slow disease, only symptomatic - movement disorder: non-Rx PT/OT/ST/swallowing - emotional: Rx for depression, irritability, anxiety, etc - counseling: open discussion w/ fam about end of life issues, revisit regularly as disease progresses |
|
|
Multiple Sclerosis
|
= autoimmune demyelination and degeneration of axons
Epi: leading non-traumatic cause of disability in adults, F>M (men later, more progressive), 15-50y (exposure thought to be around 10-15y), incidence ↑farther from equator, 8.5-10K diagnosed/y, 10x ↑ risk among relatives, higher in twins (variable) Possible causes/association: - Environmental: Vit D deficiency - Viral: EBV - Genetic: HLA-DRB1 gene (IL-7 receptor), certain populations protected (N. Americans, Eskimos, Lapps, Hungarian gypsies), 10x FHx risk, a/w thyroiditis & psoriasis Types: - relapsing remitting: 80%, typically presenting form, due to de/remyelination of axons. W/o dz altering therapy 50% becomes secondary progressive in 10y - primary progressive 10%: not responsive to treatment - secondary progressive: more common in men, occurs after period of relapsing/remitting - progressive relapsing Prognosis: (at 15y) 80% functionally impaired, 50% unable to walk, 70% unable to work. Overall 10y reduction in lifespan |
|
|
Charcot’s triad
|
3 signs of MS (though not unique to MS)
-nystagmus - intention tremor - telegraphic speech Also noted reduce memory, difficultly learning |
|
|
Clinical syndromes of MS
|
Charcot’s triad: nystagmus, intention tremor, telegraphic speech
Others: - bowel/bladder problems - cognitive difficulties - depression, fatigue, sexual dysfunction - muscle rigidity, stiffness, spasticity (ataxia, gait abnormalities), weakness or poor coordination - numbness and tingling/Lhermitte’s sign (positive symptom from hyperexcitable response to demyelination) - permanent loss of function (from persistent conduction bloct Cause: - due to loss of conduction following segmental demyelination (low # of exposed Na channels on exposed axon), reduced safety factor (current dispersed at nodes) - worsened by inflammation, elevation of temperature, increased stimulation - waning of symptoms a/w remyelination of axons (new internodes are shorter, nodes occur at areas previously myelinated, fewer Na channels) |
|
|
Workup of MS
|
Dx: (>2 attacks + lesions—disseminated in time and space)
- clinical symptoms: Charcot’s, other neuromuscular signs (esp wax/waning), worsening symptoms w/ heat (bath) - MRI: demyelinated/inflammatory lesions (new-Gd/healing-T2/old irreversible damage-T1) - CSF eval: ↑IgG, oligoclonal - visual/auditory/somatosensory evoke potentials of nerve conduction Tx: Plan: treat relapses, manage symptoms/provide support, modify/reduce relapses, delay progression to disability, facility acceptable QoL Rx: steroids, interferon injections (reduce peripheral T cells/activation), glatiramer acetate (mimics myelin antigen, sequesters self-reactive T-cells), natalizumab (prevents T-cell crossing of BBB), fingolimod (block T-cell exit from lymph nodes) |
|
|
Dementia
|
= chronic and severe acquired deterioration of selective mental functions
- Characterized by: acquired memory impairment w/ impairment of (1+): language (aphasia), visuospatial skills (apraxia), identification skills (agnosia), executive skills (planning, sequencing, goal-drive behavior) - maybe be produced by a wide variety of diseases depending on cortical or subcortical involvement, resulting in decreased neurologic reserve (sensitive to delirium) Prognosis: - progressive (AD, LDB), stepwise (vascular/successive strokes), relatively table (traumatic injury) - reversible or irreversible - severity based on functional impairment |
|
|
Delirium
|
= transient global disorder of impaired attention with clouding of consciousness
Clinical: waxing/waning of attention, inability to sustain/direct/appropriately shift attention resources, difficulty concentrating/easily distracted, variability in level of arousal (wandering, agitated, sleepy, hypoactive-seems like depression), visual hallucinations, make incoherent statements, autonomic instability (swing in vitals), a/w hospital M&M Causes: - I WATCH DEATH: infection, withdrawal, trauma, CNS pathology, hypoxia, Deficiency of vitamins, Endocrinopathies, Acute vascular insult, toxins, heavy metals - VITAMIN C: vascular, infectious, traumatic, autoimmune, metabolic, idiopathic, neoplastic, congenital Prognosis - typically resolves within week - can coexist w/ dementia |
|
|
Differentiating between common neurodegenerative disorders
|
Clinical history: look for characteristic deficits of diseases: repetiveness (Alzheimer’s), sleep disturbance and fluctuating symptoms (Lewy Body Dementia), behavior issues (Fronto-temporal)
Questionnaires: instrumental activities of daily living questionnaire (eval ADLs), neuropsychiatric inventory questionnaire (eval mood, subjective behavior) Physical exam: mental status exam, deficits in motor or sensory function Cognitive tests: Montreal cognitive assessment Neuropsyh assessments: test cognitive and emotional function. Incl: logical memory test, word recall, Boston naming, Trails A/B, clock/cube drawing, intersecting pentagons Imaging/Labs: CT, MRI, PET/PIB, CSD tap (amyloid, tau, ApoE), genetics and other biomarkers |
|
|
Alzheimer’s Disease
|
Risk Factors: advanced age, family Hx (mostly not Mendelian), head trauma, depression, HTN, high cholesterol, high homocysteine, B12/folate deficiency, F>M
Presentation: Hx of repetitive actions, normal motor features, memory loss. Imaging: Mesial temporal atrophy Familial AD (<5%): early onset variant (<65), single gene mutations (βAPP, PS1/2) resulting in excess Aβ production, AD w/ complete penetrance Down’s AD: extra copy of βAPP on Chr 21, leads to excess Aβ and senile plaques Pathologies: - amyloid plaques: extracellular aggregations of amyloid beta peptide (Aβ) around neurons. Has toxic effect at nerve endings causing injury, swelling, dystrophy. Possibly initiates cascade of events leading to dementia. Reduced clearance by ApoE, increased amyloid synthesis by βAPP or increased amyloid breakdown to Aβ by PS1/2 may contribute -Neurofibrillary tangles: aggregates of hyperphosphorylated tau protein forming within the bodies of neurons, causing neuronal dysmorphia, disruption protein trafficking, abnormal function - brain atrophy: cell death results from above lesions causing global atrophy but especially in the cortex and basal forebrain (ACh containing cells) Management: - biomarkers (for progression/treatment): Aβ (APP degradation product, forms plaques) measured on PET ($$) or in CSF, tau or p-tau measured in CSF - Tx: no drugs able to slow/stop progression. Use symptomatic therapy to improve cognition (AChE inhibitors, NDMA receptor antagonist, Vit E, vaccination against amyloid plaque |
|
|
Dementia with Lewy Bodies
|
= midpoint on the spectrum between AD and PD, characterized by pathologic Lewy Bodies
Epi: 10-20% of all dementias, M>F, 20% increase risk w/ FHx Presentation (cognitive + motor symptoms): daily fluctuation of symptoms - memory loss, inattention, executive and visuospatial change, sleep disturbances (REM behavior disorder), visual hallucinations, delusions, depression, anxiety - parkinsonism: less tremor, more shuffling gait, reduced arm swing, rigidity, facial masking Imaging: posterior atrophy Genetics (similar risks as AD/PD): ApoE4 risk gene, presenilin (PS1/2) mutations, synuclein mutations, others Pathophys: ↓cortical ACh (like AD), ↓striatal dopamine (like PD), amyloid plaques and neurofibrillary tangles (like AD), Lewy Bodies (like PD) Tx: no way to stop progression, symptomatic tx w/ AChE inhibitors, dopaminergic (lower doses than PD b/c can potentiate hallucinations), use care w/ neuroleptics (v sensitive can become catatonic) |
|
|
Major causes of dementia
|
Alzheimer’s disease
Vascular dementia Dementia w/ Lewy Bodies Hydrocephalus Multiple Sclerosis Traumatic or anoxic brain injury Vitamin deficiency (B12, folate, thiamine) AIDS related dementia Infectious: syphilis, TB, lyme disease, herpes encephalitis, cryptococcus, toxoplasmosis, subacute-sclerosing panencephalitis, progressive multifocal leukencephalopathy, Pick’s Disease, Creutzfeldt-Jacob disease Pseudodementia (depression |
|
|
Assessing mental status
|
Psychiatric Mental Status Exam
- based on interview w/ patient, not formally structured. Get symptoms and signs -ABC STAMP LICKER: appearance, behavior, cooperation, speech, thought (form & content), affect, mood, perceptions (VAOS hallucinations), level of consciousness, insight, cognition, knowledge, endings (suicidal/homicidal), reliability Mini-Mental State Examination (MMSE) - structured, brief exam for objective date. Repeated serially to observe fluctuations in status - memory or concentration: digit span, continuous performance task |
|
|
Delirium vs. Dementia
|

|
|
|
Lewy Bodies
|

= abnormal intracellular protein aggregates of alpha-synuclein (function not know, involves intracellular transport, component of amyloid plaques in AD)
Micro: intracellujlar, w/ halo surrounding. Tend to be found in areas w/ melanin (substantia nigra), in DLB found throughout the cortex and may lack halo Diseases: - DLB: LBs are cardinal feature, found throughout the cortex - AD: not consistent feature, but sometimes found incidentally on autopsy - PD: widely distributed but preferentially found in areas w/ melanin (substantia nigra, locus coeruleus, dorsal motor nucleus of vagal nerve) - Normal aging: incidentally found on 30% of people on autopsy |
|
|
Primary progressive aphasia
|

= form of frontotemporal dementia manifesting as progressive loss of language (fluency, motor speech, or comprehension)
Semanitic variant: (fluent aphasia, semantic dementia) - loss of vocabulary w/o reduction in word speed (disconnect between word and meaning) - surface dyslexia: make mistakes in pronunciation when word does not follow normal pronunciation rules (blood →blued) - a/w lesions in the anterior temporal lobe (esp on the left), TDP pathology Non-Fluent variant (PNFA): - slowed, effortful speech: dysarthria (slurring), apraxia of speech (inability to transform conscious speech plans into motor action), agrammatism (incorrect grammar use). Progressively becomes mute - loss of work knowledge (similar to SD) - a/w lesions of the frontal and insular regions, Tau pathology Logopenic progressive (LPA): - intermittent fluency separated by pauses to find the right word - a/w lesions of the posterior temporal lobe and dorsal insular lobe. Common in AD |
|
|
Fronto-temporal dementia
|

= Dementia a/w frontal, insular and/or temporal atrophy (specific regions varies base on clinical type)
Pathology: -Tau: microtubule associated protein that become hyperphosphorylated leading to microtubule dysfunction and aggregation of tau isomers which deposit as Pick bodies (basophilic cytoplasmic inclusions, stain w/ silver). Different distribution from AD - TDP: transcriptional regulator undergoes translocation to TDP-43 causing hyperphosphorylated cytoplasmic inclusions (do not stain w/ silver) Types: Behavioral variant: FTD syndrome + F/T lobar degeneration on MRI (anterior cingulate and frontoinsular region, spreading to orbital and dorsolateral prefrontal cortex) -FTD syndrome (3+): early disinhibition, early apathy or inertia, early loss of sympathy/empathy, early preservative/ritualistic behavior, hyperorality and dietary changes, neuropsych profile w/ executive/generation deficits, sparing of memory/visuospatial functions Imaging: Primary progressive aphasia: semanitic, non-fluent, logopenic progressive asphasia (temporal atrophy) - FTD-plus: occurs in ALS (TDP-43 path) and parkinsonism syndromes (Tau patho) incl PSP, CBD |
|
|
Vascular territories of the brain
|

|
|
|
Seizure types
|

Generalized: (starts in deep brain then spreads simultaneously to all parts of the cortex
- tonic-clonic (Grand Mal) - Absence (petit mal) - myoclonic - clonic - tonic - atonic Partial/Focal onset (look very different depending on origin) - Simple partial (aura) = without impairment of consciousness - complex partial = impairment of consciousness (moves outside local areal of cortex) - secondarily generalized tonic-clonic = evolving to bilateral, convulsive seizure (spreads to all portions of the cortex) |
|
|
Simple partial seizure w/o impairment
|
= aura
Signs: - no alteration of responsiveness or consciousness - variable other symptoms (motor, sensory, autonomic, psychic) depending on location If temporal: de ja vu, olfactory or gustatory hallucinations, epigastric rising sensation (“wave”) |
|
|
Complex partial seizures w/ impairment
|
Signs:
- impaired responsiveness/consciousness - average duration 1-2 minutes - automatisms: lip smacking, repetitive arm movements - amnesia -post-ictal period - may generalize - may be misdiagnosed (frontal partial manifested like night terrors) |
|
|
Localizing lesions in Coma patients
|
Motor (limb posturing):
- arm flexion/leg extension→ upper midbrain - arm/leg extension→ lower midbrain, upper pons Respiratory (innate, not on ventilation): - cheyne stokes respiration pattern→ diffuse forebrain - central neurogenic hyperventilation (deep, rapid, <25 bpm)→ lower midbrain/upper pons - apneusis (deep, gasping inspiration w/ full pause then brief insufficient release)→ middle pontine - cluster breathing (closely grouped series of respirations then apnea)→ lower pontine - ataxic (complete irregularity of breathing)→ complete medullary Eyes: - pupillary light reflex: intact→ diffuse cortical damage/toxic effects, absent → structural damage - eye movement reflexes (oculocephalic “dolls eyes”, oculovestibular “caloric”): intact→ diffuse cortical damage, absent→ brainstem injury |
|
|
Special awareness states similar to coma
|
Locked-in: follow severe lower brainstem injury, patients are awake and alert but are quadriplegic and unable to speak, swallow or breathe. May be able to blink and have voluntary eye movements
Persistent vegetative state: patient are awake but not aware: functioning brainstem but non-functioning cortex. Intact sleep/awake cycles. Often caused by hypoxic-ischemic encephalopathy (lack of O2 and blood reduces glucose) Minimally conscious state: severely altered consciousness with minimal but present behavioral evidence of self/environmental awareness. Perform extensive assessment to confirm consciousness Catatonia: neurogenic motor immobility and stupor Psychogenic coma: patient unresponsive but w/ normal PE: flaccid symmetric muscle tone, normal reflexes, nystagmus w/ ice water, normal EEG |
|
|
Coma
|
= state of total absence of awareness of self and environment (all external stimuli)
- lack sleep/awake cycles Causes: - Structural: brainstem/ascending arousal system (trauma, stroke, hemorrhage), bilateral cerebral hemispheres (subdural hematomas, large/multiple brain tumors, increased ICP, degenerative disease) - metabolic/toxic: loss of substrate for metabolism (hypoxia, ischemia, hypoglycemia), derangement of normal physiology (hypo/hypernatremia, hyperglycemia, ongoing seizures, hypothyroidism), toxin (drugs, hypercarbia, liver failure, sepsis, meningitis) |
|
|
Brain death
|
= either: irreversible cessation of circulatory and respiratory function OR irreversible cessation of all functions of the entire brain (including the brainstem)
Common causes: stroke, intracranial hemorrhage, trauma, hypoxic ischemic encephalopathy after cardiac arrest, child abuse, motor vehicle accident - pts may have spontaneous movements in 1st 24hrs, Lazarus phenomenon after removal of mechanical ventilation Dx: (repeat clinical assessment usually required) - most document evidence of: complete loss of consciousness (coma), no brainstem reflexes, apnea (absence of spontaneous respirations) - must have clinical or neurological evidence of an acute CNS catastrophe that is compatible w/ diagnosis - exclusion of complicating medical conditions that may confound assessment (not severe electrolyte, acid-base, or endocrine disturbances) - no drug intoxication or poisoning - core temp >=32C (90F) Confirmatory tests: cerebral angiography (no intracerebral filling), EEG (no reactivity in response to any stimuli), transcranial US (reversal or absent flow), isotope angiography (no uptake, “hollow skull”) Document: etiology/irreversibility of condition, absence of brainstem reflexes, absence of response to pain, repeat exam, absence of respiration (PCO2 >60mmHg), justification/documentation of any confirmatory testing, time of death (when last test is complete) |
|
|
Sensory Neurons
|
- originate at the dorsal root ganglia (outside spinal cord), then ascend in the dorsal column/medial lemniscus or spinothalamic tracts
Types: - A-alpha (I): 13-20 μm, myelinated, limb proprioception - A-beta (II): 6-12 μm, myelinated, limb proprioception, vibration, pressure - A-delta (III): 1-5 μm, myelinated, mechanical sharp pain - C (IV): 0.2-1.5 μm, not myelinated, thermal pain, mechanical burning pain |
|
|
Polyneuropathy
|
= dysfunction or disease affecting many or all of the peripheral nerves (motor, sensory, autonomic)
Ways to differentiate causes: Temporality: - acute: GBS, porphyria, vasculitis, drugs (vincristine, nitrofurantoin), toxins (arsenic, thallium) - chronic or relapsing/remitting: chronic inflammatory demyelinating polyneuropathy (CIDP), intermittent exposure to toxins - stepwise: mononeuropathy multiplex Fibers involved: - primarily motor: GBS, porphyria, Charcot Marie Tooth disease - primarily sensory: paraneoplastic effect on dorsal root ganglia, Sjrogen’s, B6 intoxication - primarily small fiber: DM, amyloidosis, toxins (alcohol), drugs, hypertriglyceridemia, hereditary sensory neuropathy (HSN), HIV, Tangier’s disease, Fabry’s disease, idiopathic causes Pattern of manifestation/progression: - distal initially: GBS - asymmetric: mononeuropathy multiplex, superimposed radiculopathy, variant CID |
|
|
Symptoms of polyneuropathy
|
Positive (generated from low threshold/heightened excitability of neurons—typically from demyelinating neuropathy
- Sensory: paresthesia (jabbing/shooting), tingling, pain, cutaneous hyperesthesia - motor: cramps, fasiculations, myokymia - autonomic: diarrhea, excessive sweating, HTN, flushing Negative (due to neuronal loss of function—typical from axonal neuropathy - sensory: lack of sensation, foot ulceration, loss of balance, gait disturbance (sensory ataxia), postural tremor - motor: weakness, atrophy, fatigue, hypotonia - autonomic: orthostatic HTN, arrhythmia, impotence, constipation, decreased sweating, urinary retention, gastroparesis, dry mouth, dry eyes, retrograde ejaculation |
|
|
Diabetic peripheral neuropathy
|
= signs/symptoms of peripheral nerve dysfunction in people w/ DM after exclusion of other causes
Epi: 30% of DM patients will develop. High risk w/: poor glycemic control, HTN, advanced age, long duration of DM dyslipidemia, tobacco use, alcohol abuse, HLA-DR3/4 - Axonal neuropathy Presentation: symmetric (stocking/glove distribution), chronic distal sensory +/- autonomic neuropathy, ankle-jerk reflex - typically pansensory (occasionally selectively small or large fiber): reduced thermal thresholds, pain, reduced vibration sense, paresthesias, absent ankle reflexes, discomfort (pain, burning), mild weakness - autonomic symptoms: increased peripheral blood flow, postural hypotension (reduced sympathetic tone), arrhythmias (cardiac deinnervation), early gastric satiety - neurogenic osteoarthropathy (Charcot’s joints): calcification and joint dysfunction around the navicular bone Work-up: nerve conduction studies (NCS), electromyelographs (EMG), HbA1C, GTT, autonomic testing Tx: control DM, pain management, foot care |
|
|
Demyelinating neuropathies
|
Pathogenesis: typically due to inflammatory attack on myelin sheath. Neurons undergo de- and re-myelination resulting in slowed conduction velocity on nerve conduction study (NCS)
Presentation: motor predominance (weakness, normal bulk, areflexia, hypertrophic nerves Types: Charcot Marie Tooth Disease (most common, generalized), Guillain Barre syndrome (acute, segmental), chronic inflammatory demyelinating polyneuropathy (chronic |
|
|
Guillain Barre Syndrome
|
= acute inflammatory demyelinating polyneuropathy
Epi: most common cause of acute generalized paralysis, 0.6-1.9/100K/y globally, peaks in young adults and 50-80’s Risks: preceding illness (campylobacter jejuni, CMV, EBV, HSV, influenza, mycoplasma), immunization, recent surgery, renal transplant, systemic illness (Hodgkin’s, SLE, HIV) Pathogenesis: autoimmune T-cells attack peripheral myelin Presentation: - weakness, areflexia, mild sensory symptoms, CN VII involvement, autonomic dysfunction - ascending symptoms from lower extremities (50%) Prognosis: - symptoms usually <1mo, majority make good recovery w/in 1yr (15% no residual symptoms, 50-60% near normal function, 10% residual weakness), mortality 2.4-6.4% (w/o ventilation) 15-30% (w/ ventilation). Poor if distal compound muscle action potential (CMAP) <20% at 3-5wk Dx: - mild ↑ESR, abn LFTs, occasional ↓Na, CSF normal at 24h protein after 1wk. - NCS/EMG: delayed/absent F waves, segmental demyelination Tx: IVIg, plasma exchange. Steroids not recommended, ICU admission if necessary |
|
|
Poliomyelitis
|
= disease resulting from infection w/ a virus that preferentially targets the ventral horn of the spinal cord (motor area) and cranial nerve nuclei
Causative viruses: Poliovirus 1,2,3, Coxsackie virus group A type &, echonovirus 6, Enterovirus 70,71, Japanese encephalitis virus, rabies virus, West Nile virus Presentation: - 1-2d non-specific viral prodrone: majority recover, minority get meningoencephalitis +/- paralysis 3-10d later. - Paralytic poliomyelitis: begins w/ myalgias and cramps then rapidly progressive paralysis (peak 48h). Typically asymmetric, confined to limbs and trunk (predisposition for lumbosacral segments). Affect limbs: flaccid and areflexic, minority have only bulbar weakness (10-15%) - CN 7,9,10 most affect, CN3,4,6 usually spared. Autonomic dysfunction if brainstem tegmentum and hypothalamus affected Dx: ↑CSF protein, CSF pleocytosis w/ lymphocytic predominance, serum Abs, stool/throat viral cultures, CSF IgM or RNA for WNV |
|
|
Spinal Muscular Atrophy (SMA)
|
= progressive hereditary disease of the anterior horn cells and select cranial motor nuclei
- phenotypically heterogeneous, progression/prognosis correlate w/ age of onset Genetics: deletion of survival motor neuron 1 gene (SMN1) on Chr 5. Mostly homozygous, some heterozygous. Severity depends on # of SMN2 copies (more =↓severity) which in normal has no function (takes over in disease state) Types: SMA1 (“Infantile” or “Werdnig-Hoffman Disease”): most common, onset <6mo, death by 2 w/o mechanical ventilation - hypotonic (floppy) infant, proximal weakness, areflexia, never able to sit, mild facial weakness, eye movement spared, tongue fasciculations, abdominal breathing, weak cry, poor suck, respiratory failure from intercostal weakness, no intellectual impairement SMA2 (Intermediate): Onset 6-18mo, 98% survival @5y, 66% @25y - ability to sit independently but never walk, tongue fasciculations, areflexia, proximal weakness, postural hand tremor, kyphoscoliosis, joint contractures SMA3 (“Juvenile” or “Kugelberg-Welander Disease”): onset >18mo, life expectancy 60y-normal - can stand & walk, proximal weakness, hand tremor, areflexia, tongue fasciculations, notable limb fasciculations SMA4 (Adult): rare, onset 20-30’s. Genotypically heterogeneous, presents w/ proximal weakness |
|
|
Kennedy Disease
|
= X-linked Bulbospinal Muscular Atrophy
Course: progressive bulbar and proximal weakness (mean onset 44) → wheelchair by age 61 Pathogenesis: mutation of androgen receptor gene leading to androgen insensitivity Other symptoms: gynecomastia, impotence, testicular atrophy, infertility, increased incidence of DM |
|
|
Amyotrophic Lateral Sclerosis (ALS)
|
= progressive dysfunction upper and lower voluntary motor system
Epi: incidence 1/100K, onset 50%, >90% sporadic (5-10% familial, AD), M>F, 30K in US Signs: (vary between individuals, begin focal then spread locally/regionally) - UMN: spasticity, hyperreflexia, jaw jerk, Hoffmann’s sign, Babinski’s sign - LMN: weakness (asymmetric, bulbar), atrophy, hyporeflexia, muscle cramps, fasciculations - non-motor: pseudobulbar affect (emotional lability), frontotemporal dementia (5-20%), depression, anxiety Pathology: degeneration and death of motor neurons (cause unknown) - UMN degeneration of Betz cells in corticospinal tract and LNM degeneration of anterior horn cells - sparing of CN3,4,6 and Onuf’s nucleus (bowel, bladder function) Prognosis (variable): 50% mortality 3-5y (occasionally live decades), most common cause of death is respiratory failure (restrictive lung disease) Management: Tx: Riluzole (↑survival 2-3mo for pts w/ 3yrs), dextromethorphan/quinidine for pseudobulbar affect, baclofen/tizanidine for spasticity, NSAIDs/narcotics for pain, antidepressants. Also PT, OT, ST, devices (chair, braces, cervical collar, respiratory, suction), nutritional changes |
|
|
Sleep
|
Definition: reversible biobehavoiral state of perceptual disengagement and unresponsiveness to the environment w/ physiologically defined patter of neural and muscle activity. Characterized physiologically, behaviorally, subjectively.
Functions: brain/body restoration (tissue/protein/glycogen synthesis), thermo regulation, energy conservation, non-adrenergic regulation, memory consolidation, mood regulation, brain development, etc Stages: drowsy (alpha waves), stage 1 (theta waves), stage 2 (K copmlexes), delta slee (deepest), REM (sawtooth waves |
|
|
Sleep architecture
|
Normal young/middle age: abundant stage 3/4 early in sleep. REM occurs about every 1.5hrs (20-25% of total)
Older adult: decline in deep sleep stages, more fragmentation (waking at night), REM fairly stable (20-25% of total) Infants: more REM sleep than adults |
|
|
Classification of sleep disorders
|
Not mutually exclusive:
A. Disorders involving daytime fatigue, tiredness, and sleepiness B. Disorders involving inability to sleep C. Disorders involving events occurring during sleep |
|
|
Insomnia
|
= most prevalent sleep symptom
- difficulty maintaining sleep is the most common complaint: increases with age (50% by age 60) - difficulty falling asleep: no age-related prevelance - waking up too early (an unable to fall back asleep) - sedative/hypnotic use: F>M Treatments (differ by T1/2, dose, class): BZDx, non-BZDx, MT agnoists. Also sleep hygiene, relaxation, exercise, etc - benefits: usually work, better then OTC/herbals, range of T1/2 and dosing options, probably safter/more effective than low dose anti-depressants - risks: tolerance, falls/balance problems, odd behaviors, possible ↑mortality/cancer risk Pathogenesis model (3P’s) - predisposing factors: anxiety (worry/stress about sleep), depression, personality, ↓homeostatic sleep drive - precipitating factors: medical/psych illness, medications, shift work, stressful life events - perpetuating factors: counterproductive efforts, poor sleep hygiene, psych conditioning |
|
|
Sleep control
|
- balance between sleep drive and circadian alert signal. Both build as day progresses
|
|
|
Narcolepsy
|
= inability to sustain wakefulness throughout the day (hypersomnia) due to poorly consolidated states of thalamocortical arousal (wake-sleep instability): intermittent REM sleep spells often triggered by emotion, fragmented sleep, but normal total amount of sleep.
Hallmarks: sleepiness (sensitive for narcolepsy), cataplexy (sudden muscle atonia, specific for narcolepsy, 64-90% of patients), sleep paralysis (“weight on chest”), hypnagogic/hypnopompic hallucinations (fall into/out of dream). May also have REM behavior disorder (dream enactment (specific if <35)) Causes: H1N1 infection or other seasonal infectious agent Pathogenesis: loss of hypocretin (excitatory peptide) producing cells in the thalamus (may be protein or receptor mutation), resulting in loss of excitation to areas required to maintain awareness (basal forebrain, tuberomammillary nucleus, ventral tegmental area, locus coeruleus, others) and loss of inhibition to REM sleep Dx: - polysomnography: short nocturnal REM latency (REM periods <60min into sleep,), fall asleep fast (<8min), disruptive nocturnal sleep (↑stage 1, ↑waking, ↓sleep efficiency), periodic leg movement (>10/h, 40% of cataplexy pts) - Labs: HLA-DQB1 0602/15, absent CSF hypocretin Tx: regular scheduled naps, exercise Rx: modenafil (provigil), methylphenidate, amphetamines, sodium oxybate (date-rape drug), selegiline (MAO-I), SNRI’s, TCAs, SSRIs |
|
|
Restless Leg Syndrome (RLS)
|
Dx criteria (75% sens/spec):
- urge to move legs, usually accompanied by uncomfortable leg sensations - onset or worsening of symptoms at rest or inactivity (sitting/lying) - relief w/ movement partial or total relief w/ walking or stretching - worsening symptoms in evening/night Epi: 10% of US, F>M, Caucasian/Scandinavian predominance, often AD familial (SNP’s identified w/ dose-dependent relationship w/ PLM, anemia), many are anemic/low ferritin A/w: Fe deficiency, pregnancy, VitB12 deficiency, periodic limb movements (during sleep, avg 22.7s interval, peak at 1am) mood/anxiety disorder, panic disorder, CVD (HTN esp PLM >30/h) Pathogenesis: disorder of emotional motor system (ventromedial medulla which sets spinal reflexes) resulting in disinhibition of spinal reflexes. Sensory symptoms ascend spinothalamic tract. Fe deficiency causing abnormalities in dopamine signaling, may be causative. Workup: document 4 criteria, look for medical aggravators (antihistamines), FHx (esp women), psych eval (anxiety, depression), neuro eval (usually normal), serum Fe/Ferritin, B12, TSH, ambulatory actigraphy for PLMs Tx: - counterstimuli, Fe repletion, dopaminergics (ropinirole, pramipexole). Off label: opiods, anticonvulsants, BZDs |
|
|
Parasomnia
|
= undesirable physical events or experiences that occur during entry into sleep, within sleep or during arousals from sleep (nightmares, sleep walking, etc)
|
|
|
REM behavior disorder
|
= disorder of dream content and movement in which pts act out vivid dreams (commonly violent themes), have good dream recall upon waking. Predominantly elderly men
Mechanism: lesion of subdorsolateral nucleus + sufficient locomotor drive lack of atonia of skeletal muscle during sleep + presumed failure of movement suppression during sleep Complications: potentially lethal behaviors (choking/headlock of partner, throwing, punching, diving from patient) Acute form: iatrogenic (antidepressants), withdrawal (GABAnergics, ETOH, barbituates, BZDs), structural brain injury (ischemic, hemorrhage, tumor, demyelination, inflammation) pontine lesions, limbic encephalitis A/w: narcolepsy/hypocretin deficiency in young patients, α-synucleinopathies (PD, MDA, LBD) in elderly men, may be idiopathic Dx: (8.7 +/-11y after symptom onset) - REM sleep w/o atonia (polysomnography) + Hx of injurious/disruptive sleep behavior or abnormal REM sleep during PSG Tx: remove dangerous objects, beds w/ rails, remove stimulating meds, clonazepam before bed (90% response), melatonin |
|
|
Non-REM arousal disorders
|
= incomplete arousals from slow-wave sleep (stage 3/4) s in dissociation btwn awake and sleep state (behavior vs. EEG)
- typically disorders of childhood but can persist to adulthood (should consider occult causes of arousal if new symptoms in adulthood) Types: - confusion arousals: <5yo, wake up confused (appearing), return to sleep - sleep terrors: 5-7y, child has no memory of arousal, severe distress (more for parent than child unlike nightmare) - somnambulism (sleep walking): >7y Tx: safety of environment, avoid triggers (sleep deprivation), clonazepam if needed |
|
|
Nocturnal Frontal Lobe Epilepsy
|
= frontal lobe seizures that occur during sleep, often misdiagnosed as non-REM sleep disorders
Epi: mean onset 14y (1-64y), 70% M, - cluster seizures: may have 20/night but may not have every night - 1/3 have daytime events - can occur in any stage, but Stage 2 most common, so tend to occur soon after falling asleep - event recall is variable Tx: carbamazepines or other anticonvulsants, ensure safety of environment |
|
|
Worrisome features of headaches
|
Wakes the patient from sleep
Constant, worsening over months Fever Sudden onset, severe, meningismus (triad of nuchal rigidity/neck stiffenss, photophobia, headache) Neurological deficits Trauma Papilledema (often due to tumor or idiopathic intracranial HTN) |
|
|
Migraine pathophysiology
|
- unknown trigger (aspartame, chocolate, red wine, etc) causes cortical spreading depression/electrical disturbance that spread across the cortex.
- If electrical disturbance reaches the occipital lobe it will cause auras, which spread across the visual field at the same rate. - cortical spreading triggers the trigeminal nerve which stimulates release of substance P and other inflammatory mediators causing vasodilation and inflammation→ sensitizes trigeminal nerve to sensory stimuli - cortical spreading triggers the trigeminal nucleus caudalis which forwards pain signals to the thalamus and sensory cortex. - Role of vasodilation of the extracranial arteries is believed to be significant |
|
|
Common migraine
|
5 attacks fulfilling criteria:
- headache lasting 4-72hrs (untreated or unsuccessfully treated) - Headache w/ 2+ characteristics: unilateral location (can become bilateral), pulsating quality, moderate or severe pain intensity, aggravation by or causing avoidance or routine physical activity - During headache N/V or photo/phonophobia/sensitivity to smel |
|
|
Classic migraine
|
- differentiated from common migraine by present of auras
- reversible neurologic deficit ( >5min but <60min) - types of aura: visual (positive or negative features), dysphagia, hemisensory deficit - cortical spreading depression of leao: inciting event in the occipital cortex for visual aura causes lack of blood flow there. Elecctrical/vascular disturbance spreads 3-5min. Oligemia (low, slow blood flow) causes neuronal change that can induce positive or negative visual phenomena. |
|
|
Hemiplegic migraine
|
- migrainous symptoms with fully reversible motor weakness and aphasia (often). basilar symptoms often present as ataxia
Familial Hemiplegic Migraine - present in 1/2 degree relative, - defects: CACNA1A channel (Chr 19, FHM1), ATP1A2 gene (Chr 1, FHM2), SCN1A gene (Chr 2, FHM3) Sporadic Hemiplegic migraine: No family history |
|
|
Basilar Migraine
|
= likely due to basilar artery spasm
Aura consisting of 2+ fully reversible symptoms, w/o motor weakness - dysarthria - vertigo - tinnitus - hypacusia - diplopia - visual symptoms simultaneously in both temporal and nasal fields of both eyes - ataxia (basilar artery supplies the cerebellum) - decreased level of consciousness: raphe nuclei affected by basilar artery lesion - simultaneous bilateral parathesias |
|
|
Obscure migraine variants
|
Cyclical vomiting (w/o headache)
- 1h to 5d, vomiting >4x/h for 1 hr - symptom free between attacks Benign Paroxysmal Vertigo of childhood - multiple episodes of severe vertigo, occurring w/o warning and resolving spontaneously after minutes to hours - normal neuro exam, audiometric and vestibular function between attacks, normal EEG - often a/w nystagmus or vomiting, unilateral throbbing headache may occur w/ some attacks |
|
|
Migraine Therapy
|
Abortive Therapy: Triptans (use as soon as headache starts, good for up to 6 migraines/mo)
- variable absorption and T1/2 - Avoid w/: vascular comorbidities, hemiplegic migraines, basilar migraine, pregnancy (constricts placental vessels) Alternative abortive strategies: - NSAIDs, Triptan/NSAIDs combo, tramadol, antihistamines (pregnancy), Opiates Prophylaxis - Vit B2, magnesium, anticonvulsant prophylaxis (if has seizures), anti-HTN (beta-blockers), TCAs, IV DHE or opiates (for continuous migraines) Non-pharm prophylaxis - neck/spinal manipulation, pulsating electromagnetic fields, TENS, neck exercises, spinal mobilization, regular lifestyle/sleep/meals/exercis |
|
|
Sinus headache
|
= frontal headache w/ pain in 1+ regions of the face, ears or teeth
- clinical, nasal endoscopic, CT and/or MRI evidence of acute/subacute rhinosinusitis - typically resolves w/in 7 days after remission or successful treatment of rhinosinusitis - Clinical evidence: purulence, nasal obstruction, hyposmia/anosmia, headache and facial pain (less likely), nasal obstruction/post-nasal drip (more likely) |
|
|
Medication overuse headache (MOH)
|
= occur when analgesics (or caffeine) are taken chronically
- classically occur when patients has underlying headache disorder that “transforms” from an episodic condition to a chronic, daily headache Criteria: - headache present >15d/mo - regular overuse >3mo - headache has developed or worsened during med overuse - headache resolves or reverts to previous pattern w/in 2mo after discontinuing medication Tx: reduce offending agent, prophylaxis for underlying headache condition, consider IV abortive therapy if patient can’t tolerate pain |
|
|
Tension headache
|
= most common primary headache, radiates from lower back fo the head, neck, eyes, or other muscle groups
Criteria: - 10 episodes of: episodic: <180/y (<15/mo) or chronic: >15d/mo - lasts 30min to 7days - 2+ pain characteristics: pressing/tightening (non-pulsing), bilateral location, mild/moderate intensity w/o aggravation from physical activity - no N/V or photo/phonophobia |
|
|
Cluster Headache
|
= trigeminal autonomic cephalgia, usually along the distribution of the V1 facial nerve
Criteria: >5 attacks w/ - severe unilateral orbital, supraorbilatal, and/or temporal pain lasting 15-180min or until treated - Additional signs: conjunctival lacrimation, lacrimation, nasal congestion, rhinorrhea, forehead and facial sweating, miosis, ptosis, eyelid edema Frequency: 1 every other day up to 8/day Tx: oxygen, abort w/ analgesics or triptans, sumatriptan injection/nasal spray, dihydroergotamine, ergotamine, zolmitriptan, intranasal lidocaine, greater occipital nerve blockade, verapamil |
|
|
Chronic Paroxysmal hemicranias
|
= chronic pain in half the head
Criteria: 50 attacks fulfilling - severe unilateral orbital or superorbital and/or temporal pain always on the same side lasting 2-45min - >5/d for more than half of the time (no periods w/ lower frequency may occur) - no predilection for night attacks - accompanied by lacrimation (62%), followed by nasal congestion (42%), conjunctival injection and rhinorrhea (36%) and pstosis (33%) |
|
|
Hemicrania continua
|
= continuous unilateral headache
- remitting (15%) and non-remitting (85%) forms - pain is 24/7. Some autonomic symptoms exacerbations w/ coexisting migraines or cluster headaches Respond to Indomethacin as an abortive |
|
|
5 ocular vital signs
|
Visual acuity: myopia, hyperopia, astigmatism, presbyopia
Pupils: CNIII palsy, horner’s syndrome, tear drop, anisocoria, Argyll Robertson pupil of syphilus Intraocular pressure: glaucoma Ocular motility/position: exotropia, esotropia, CN 3, 4,6 palsy Visual Fields: homonymous hemianopsia, bitemporal hemianopsia, others |
|
|
Causes of red eye
|
External: epibulbar + palpebral hyperemia
- infection: --- viral (clear discharge, preauricular node, URI prodrone): adenovirus (super contagious,), HSV (mucocutaneous impetigo, dendritic corneal ulcer, inflammatory blindness, NO steroids, antivirals), herpes zoster (usually V1 only) --- bacterial/fungal (bilateral, purulent discharge): N. gonococcus (hyperpurulent, BAD), staph, strep, H. Flu, pseudomonas - allergy (stingy white discharge, swollen lids, itch): atopy, hay fever (tx: decongestants, anti-hists, mast cell inhibitors, steroids) - chemical - trauma: sub-conjunctival hemorrhage (rubbing/vasalva, not serious), tear deficiency state (age, meds, RA, TEN—epidermal sloughing), corneal abrasion (deficient epithelium), corneal ulcer (infected abrasion, NO anathetics, topical Abx) Internal: epibulbar - palpebral hyperemia - inflammatory/congestive disorder of the globe: glaucoma - hyphema (bleeding in the eye/anterior chamber) - orbital congestion (Grave’s dz, orbital cellulitis, high flow carotid fistula. Impedes return flow |
|
|
Anterior Ischemic optic neuropathy
|
= ischemia of the nerve head
- epi: M=F, >50y - painless swelling of the disc (pallor months later) - a/w giant cell arteritis though also non-arteritic forms (DM, HTN) - permanent visual loss (ischemia area relate to visual field loss) |
|
|
Gaze pathways
|
Superior colliculus in the midbrain
Horizontal: parapontine reticular formation - CN6 sends connections to contralateral CN3 nucleus via MLF to make eye adduct when partner abducting - saccades done in the paramedian pontine reticular formation: contralateral nucleus then uses MLF - pursuits use the ipsilateral pathway - convergence is the only horizontal movement that does not go through CN6 (all CN3) - if MLF is knocked out bilaterally think MS. May be a/w nystagmus |
|
|
Giant cell arteritis
|
- primary vasculitis of the branches of the internal carotid
Signs (from ischemia) >50y - systemic : weightloss, fatigue, headaches (temporal), scalp tenderness, jaw claudication, high ESR/CRP/Platelets - ocular: sudden, transient monocular vision loss (ischemic optic neuropathy), choroidal ischemia, central retinal artery occlusion (looks like retinal swelling—cherry red spot), ocular ischemia, diplopia (EOM/CN ischemia), cerebral ischemia R/o emboli (carotid U/S, CTA, EKG, cardiac echo) Work-up: inflammatory markers, start steroids immediately, arterial biopsy w/in 2 weeks to confirm |
|
|
Optic neuritis
|

= inflammation of the optic nerve
- F:M 3:1, age 15-35, - idiopathic demyelinating optic neuritis is most common - presentation: pain on eye movement, central vision loss - normal (behind eye) or swollen disc (near eye), months later will have pallor from dead neurons - a/w multiple sclerosis - spontaneous improvement (40% redundancy, so vision despite loss of neurons) |
|

|
Cataract
- leading cause of blindness worldwide Risks: age, corticosteroids use, UVA/B exposure, HTN, obesity, ionizing radiation, diabetes, excessive alcohol, previous eye surgery, FHx of cataracts Pathophys: - congenital: rubella - age related: lens fibers degenerate and lens clouds - subcapsular: a/w systemic disease (diabetes: sugars converted to insoluble sugar alcohols) or steroid use Rx: lens replacement |
|
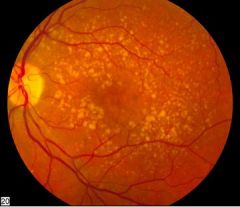
|
Macular degeration
- leading cause of blindness for >65y Risks: age>60, smoking, UVA/B exposure, HTN, heart disease, FHx, Caucasian, F>M, light iris color, poor nutrition Pathophys: - dry: retinal pigment epithelium stops recycling by products quickly allowing waste to accumulate in the subretina (drusen spots: yellow around the macula) causing detachment - wet: accumulated drusen causes defects in the retinal pigment epithelium causing neovascularization from the choroid leading to hemorrhage Fundoscopic: drusen accumulation, retinal pigment epithelium atrophy, choroidal neovascularlization Prevention: smoking cessation, take vitamin (lutein, zeaxanthin, carotene, zinc, copper, C/E), UVA/B sunglasses, eye exams (patient sees central grid distortion due to tenting of the macula), good nutrition, HTN/CV dz control |
|

|
Glaucoma
= progressive, bilateral optic neuropathy - second most common cause of blindness in the US (2/3 open, 1/3 closed) Risks: family Hx (6x), African American, Hispanic, advanced age, intraocular pressure >21 (some may be normotensive due to anatomical variance) Pathophys: insufficient fluid drainage (through trabecular meshwork) relative to production. In closed angle the path around the iris is obstructed resulting in rapid, painful glaucoma Fundoscopic: enlarged cup/disc ratio (>0.3) indicating loss of optic nerve fibers, recession of optic nerve, increased IOP, notching of optic cup, splinter and flame/drance hemorrhages (acute nerve loss), progressive peripheral vision loss Screening: eye exam for all at age 40 (30 if FHx) and high risk groups Rx: alpha/beta agonists, surgery |
|

|
Diabetic retinopathy
- leading cause of new-onset adult blindness in the US Risks: long term poor diabetes controlled (HbA1C >7%, 70% w/in 10yrs), uncontrolled HTN, renal disease, pregnancy, anemia Pathophys: - non-proliferative: ↑glucose/metabolites → vascular pericyte destruction → microaneurysms → retinal hemorrhage, edema - proliferative: poor blood supply due to clots → ischemia → neovascularization (VEGF) → vitreous hemorrhage, retinal detachment Fundoscopic: yellow lipid exudates, microaneurysms, intraretinal hemorrhages Prevention/screening: diabetes control, T1DM screening 3-5y after diagnosis then yearly, T2DM at diagnosis then yearly Rx: laser ablation of microaneurysm, anti-VEGF intraocular injections |
|
|
Spectrum of consciousness
|

|

