![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
150 Cards in this Set
- Front
- Back
|
Neurotransmission (synaptic transmission)
|
- Process by which neurotransmitters are released by a presynaptic neuron and bind/activate receptors on the postsynaptic neuron.
|
|
|
Why bother with going from an electrical to a chemical to an electrical signal?
|
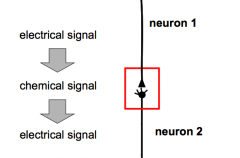
2. Regeneration
2. Increasing specificity (different types of neurotransmitters) 3. Modulation of signal (environment makes a difference) 4. Amplification: can make the signal bigger 5. Integration of many inputs |
|
|
What are the two types of synapses?
|
- electrical synapse
- chemical synapse |
|
|
Who discovered the chemical synapse and how?
|
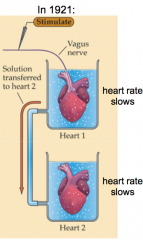
- Otto Loewi won the Nobel Prize for demonstrating chemical neurotransmission
- Heart rate slows if you stimulate the heart with the vagus nerve - If the solution from heart 1 has a similar effect on heart 2 without stimulation of heart 2, something must be released into the cell |
|
|
Loewi Expt Part 2 (make sure you can explain this)
|
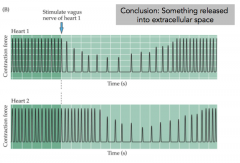
|
|
|
Synaptic cleft
|
- space between the pre- and postsynaptic cleft (~20nm wide)
|
|
|
Synaptic vesicles
|
- the things that hold NT and release it to the synaptic cleft
|
|
|
Presynaptic
|
- neuron before the synaptic cleft
|
|
|
Postsynaptic
|
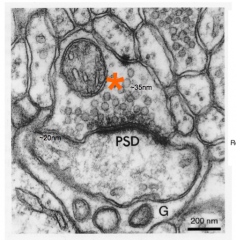
- neuron after the synaptic cleft
|
|
|
Synaptic delay?
|
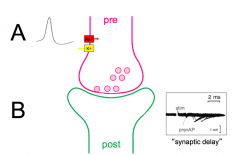
- how do you figure out what happens?
- synaptic delay - delay between the AP in the presynaptic neuron and the response in the postsynaptic neuron |
|
|
Bernard Katz background
|
- Never seen an EM
- Knows neurons are separate - do know that it's a chemical NT - Work was done at the NMJ |
|
|
Neuromuscular Junction vs Two Neurons
|
- Presynaptic and postsynaptic neurons in the central nervous system
- excitatory postsynaptic potential (EPSP): CNS Neuromuscular junction - Motor neuron and muscle (end plate) - End Plate Potential (EPP) |
|
|
I don't know...the next few notecards will deal with this
|
|
|
How did Katz et al. show that the postsynaptic response is dependent on extracellular [Ca2+]? (Point 1)
|
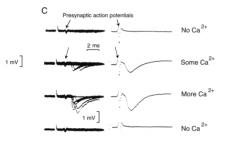
- Removing Ca2+ from the cell = no signal
- Adding Ca2+ = BIG response - Ca2+ can modulate the signal - NOTE: depolarization on inside looks like hyperpolarization |
|
|
What is the precise dependence upon exterior [Ca2+]? (Point 1)
|
- EPP is proportional to exterior [Ca2+]?
- Dodge-Rahamimoff Relation - Response = (exterior [Ca])^3.78 - Ca2+ cooperativity: if you have on Ca2+ it makes release more likely and therefore there must be multiple binding sites - IT'S NOT LINEAR!!! |
|
|
Is the postsynaptic response dependent upon presynaptic voltage?
|
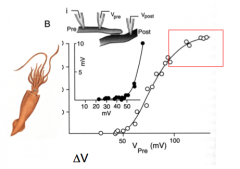
- Prep: Giant squid axon
- bottom is change in voltage away from rest - at -50mV, VG Na+ open - The square box means something - I would ask - Red box = nearing the tail for +40mV (?) |
|
|
In what quantity/form is NT released? (Is release of NT discrete or continuous?)
|
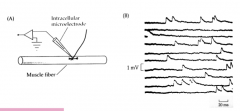
- discrete: n number of "packets" or quanta
- Katz and colleagues observed discrete postsynaptic events or minis - saw random blips that were all around about the same size - no graded process - miniEPP = not an AP, but smaller depolarizations - Did a Poisson analysis to compare to how a discrete process should behave CONCLUSION: NT is released in discrete packets! (in NMJ and later was realized in the CNS) |
|
|
Mini EPP
|
- mEPP - not an AP, but smaller depolarizations
|
|
|
Evidence for NT release
|
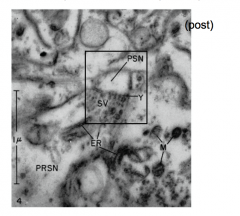
- De Robertis and Bennet
- showed synaptic vesicles in the presynaptic terminal |
|
|
More evidence for NT release
|
1981: Heuser and Reese showed vesicles "caught in the act"
- Vesicles exist in the presynaptic terminal and appear to be storage vesicles for NT. |
|
|
Does [Ca2+] get into the presynaptic terminal upon depolarization? (we've ony shown that the postsynaptic response is dependent upon extracellular [Ca2+])
|
- Can measure Ca2+ current (I)
- Block Ca2+ - Visualize Ca2+ using indicator dyes - Increased internal [Ca2+] (uncaging expts) to show that calcium triggers release directly |
|
|
Calcium uncaging experiments (make sure that you understand this)
|
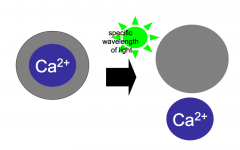
- Resting Ca2+ levels are extremely low inside neurons (because it's an important signal)
- Largely due to an army of Ca2+ buffering proteins that reside inside the cell - Exogenous Ca2+ buffers can be introduced into the cytoplasm to block increases in internal [Ca2+] - Ca2+ uncaging: synthetic Ca2+ buffer; conformation dependent on light; if you shine a light, it will release Ca2+ - uncaging experiments show that increased calcium inside presynaptic terminal triggers release |
|
|
Calcium uncaging experiments (data)
|
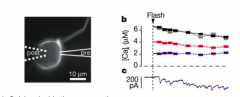
- Calcium inside the presynaptic terminal is both necessary and sufficient for release of NT
|
|
|
More Ca2+ Uncaging Experiments
|
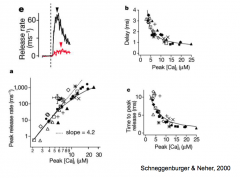
1. Calcium inside the presynaptic terminal is both necessary and sufficient for release of NT
2. Concentration of Calcium can dictate the amount of release |
|
|
Calcium current during depolarization
|
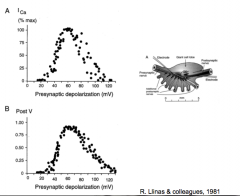
R. Llinas et al.
- increases in presynaptic voltage lead to inward calcium current in the presynaptic terminal |
|
|
Where does calcium enter the cell relative to the release site (active zone)?
|
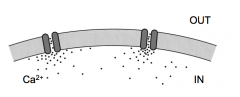
- Calcium microdomains
- Calcium enters via Calcium via channels and forms a local mcirodomain near the channel that quickly dissipates (binds to buffer) - channels located right next to vesicles |
|
|
Evidence for chemical NT release (summary slide)
|
1. Postsynaptic response is dependent on external [Ca2+]
2. Postsynaptic response is dependent upon presynaptic voltage 3. Neurotransmitter is released in discrete quanta 4. Vesicles exist in the presynaptic terminal and appear to be storage vesicles for NT 5. Calcium enters the presynaptic terminal during depolarization, can trigger release, and affects the rate of release. 6. Calcium enters the cell very close toe the release site (active zone) |
|
|
Conclusions from lecture 9
|
1. Synaptic vesicles filled with NT exist in the presynaptic terminal
2. Upon depolarization of the presynaptic terminal, Calcium rushes in at specialized calcium microdomains near the active zone (presynaptic machinery) 3. Calcium binds to machinery that allows individual vesicles (representing quanta) to fuse with the presynaptic membrane and release neurotransmitter into the synaptic cleft. |
|
|
NT Release Summary
|
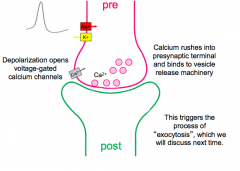
|
|
|
Returning to point 6 of Bernard Katz's experiments:
Where does Calcium enter relative to the release site (active zone)? |
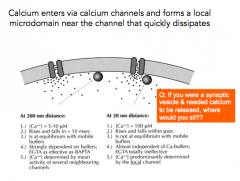
- Calcium enters via calcium channels and forms a local microdomain near the channel that quickly dissipates
- Presynaptic terminal is like a thick soup of calcium buffers Q: If you are sitting away from the presynaptic terminal, how much Calcium do you see nearby? Almost none - Calcium "buffer" binding proteins Why doesn't the cell like having Calcium around? - Calcium is dangerous and will activate the cell |
|
|
Where is the only place that Ca2+ concentration very high?
|
- very close to the Calcium channel, and even then only for a brief amount of time
- there's a SHORT amount of time before the buffers bind to calcium |
|
|
Where do synaptic vesicles sit?
|
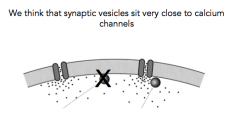
- Even a difference in 10-20 nm will result in a dramatic decrease of calcium concentration
- you'd think that vesicles are probably very close to Calcium channels |
|
|
Calcium kinetics
|
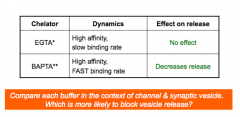
- estimated by introducing calcium chelators into the presynaptic terminal
|
|
|
Which buffer is more likely to block vesicle release?
|
- BAPTA!
|
|
|
Consequences of Calcium Microdomains
|
- ensures a local effect that is less likely to "spill over" to other release sites (high spatial specificity)
- since local [Ca] must be high; binding affinity may be low --> fast off rate (high temporal specificity) - Less likely to have an accidental event |
|
|
What are exocytosis and endocytosis?
|
- Exocytosis: release of synaptic vesicles
- Mechanisms: synaptic proteins and VG Ca2+ channels - Endocytosis: recycling of vesicles |
|
|
When does exocytosis occur?
|
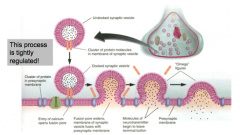
- when the membrane of the synaptic vesicle fuses with the plasma membrane
- tightly regulated - involves fusion of the synaptic vesicle membrane and the plasma membrane |
|
|
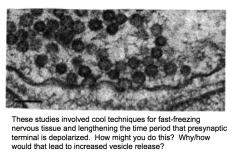
How could you design an experiment that would provide evidence for exocytosis?
|
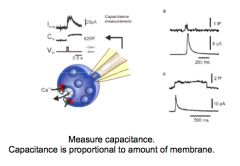
- measure capacitance; should increase with more membrane
- electron microscopy to visualize synaptic vesicles: fast freezing expts showed vesicles "caught in action" - high stimulation...eventually depletes synaptic vesicles --> would view fewer in EM - put fluorescence in vesicles and track their movement (extracellular factors) |
|
|
Heuser and Reese proposal for synaptic vesicle function
|
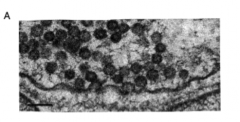
- NT packed into vesicles
- docking, priming, fusion |
|
|
Exocytosis and Capacitance measurements
|
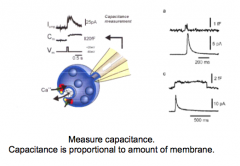
|
|
|
Exocytosis and Fluorescence
|

- extracellular factors captured in vesicles
|
|
|
Exocytosis and overstimulation
|
- Em shows that fewer synaptic vesicles are present in the presynaptic terminal following long periods of stimulation
|
|
|
What are the main steps in exocytosis?
|
- docking, priming, and fusion/NT release
|
|
|
SNARE hypothesis and Priming
|
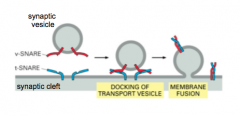
- proteins on vesicle and the membrane target
- v-SNARE: Vesicle membrane - t-SNARE: Target membrane - v-SNAREs and t-SNAREs dock vesicles at the presynaptic membrane - makes vesicle ready to fuse - 2 alpha helices come together and cause the two membranes to dock - SNAREs get it into the docked state |
|
|
Complexin
|
- Complexin holds synaptic vesicles in final primed mode
- almost about to be fused - complexin thought to keep them from fusing until it's removed (by Ca2+?) |
|
|
What is Calcium's role in exocytosis?
|
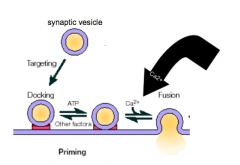
- Ca2+ is required for fusion
- likely sensor for Calcium is synaptotagmin, which is present on the vesicle - 2 Ca2+ binding motifs - Ca2+ changes conformation of synaptotagmin, allowing membranes to fuse together |
|
|
Synaptotagmin
|
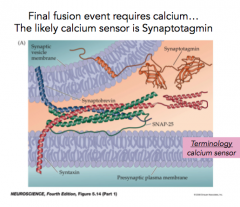
- likely Calcium sensor present on the vesicle
- has two calcium binding motifs - Calcium changes conformation of synaptotagmin, allowing the membranes to fuse together |
|
|
Fusion step, synaptotagmin, Calcium, etc.
|
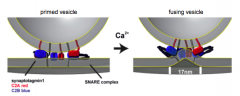
Calcium changes conformation of synaptotagmin, allowing vesicular and plasma membranes to fuse together
|
|
|
How would you predict synaptic transmission would be affected in a genetically modified "knock-out" animal that lacks synaptotagmin?
|
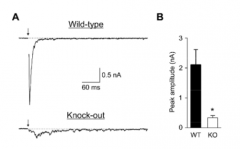
- probably no transmission in the synapses that use synaptotagmin
- Synaptotagmin is required for NT release |
|
|
Steps of Exocytosis 1 & 2
|
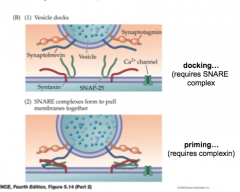
- docking...requires SNARE
- priming...requires complexin |
|
|
Steps of Exocytosis 3 & 4
|
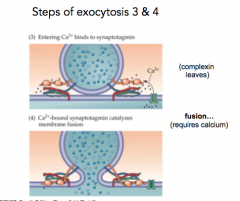
3...Ca2+ comes in an complexin leaves
4...Fusion |
|
|
NT Release Update
|
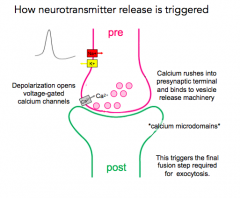
|
|
|
Do VG Ca2+ channels inactivate?
|
- No. The signal coming in is already regulated by frequency and duration of action potentials.
- Release is mediated typically by N, P/Q, and R-type Calcium channels, which have little/slow inactivation. |
|
|
What is one cost of having vesicles so ready to go?
|
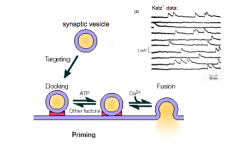
- even a small amount of calcium will start releasing 1-2 vesicles
- mini events occur - mini events occur when a primed vesicle fuses in the absence of Calcium (probably) |
|
|
What happens to NT during exocytosis/endocytosis?
|
- extracellular volume is so large that essentially all molecules of NT are ejected from the vesicle
- NT doesn't re-enter the vesicle during endocytosis - Empty vesicles are instead refilled after they are endocytosed/recycled |
|
|
Overview of synaptic vesicle cycle
|
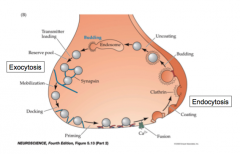
|
|
|
"Clathrin-coated pits"
|
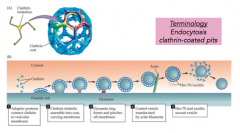
- cover and curve parts of membrane targeted for endocytosis
- individual proteins that form a skeleton and form the circular form - dynamin seals the vesicle |
|
|
Dynamin
|
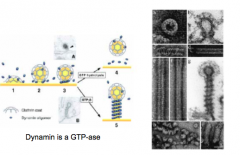
- dynamin proteins assemble around the neck of coated vesicle and lead to pinching off
- Dynamin is a GTP-ase, aka GTP dependent |
|
|
Endocytosis
|
- recycles the synaptic vesicle back into the presynaptic terminal
- clathrin may target the pieces covered with teh synaptic vesicle proteins |
|
|
How does endocytosis affect capacitance?
|
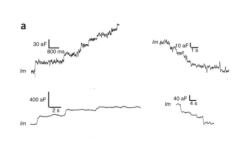
- endocytosis decreases capacitance (getting rid of membrane)
|
|
|
What are small, transient increases in capacitance?
|
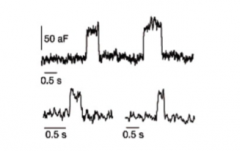
- appear quantal
- Kiss and run model of NT release |
|
|
Kiss & Run vs classic NT release
|
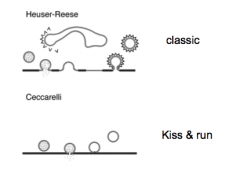
- Ask her about this too.
|
|
|
Vesicle Recycling
|
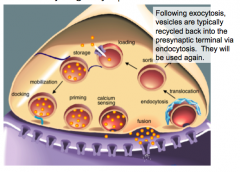
|
|
|
Reserve pool:
|
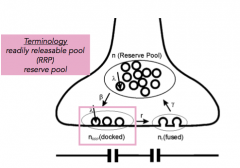
- pool of vesicles not currently primed and docked
- back up |
|
|
Readily Releasable Pool (RRP)
|
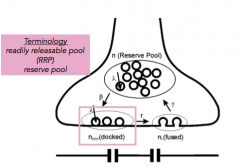
- pool of vesicles primed and at the docking site, waiting for an influx of calcium.
|
|
|
Specificity of NT
|
- one synaptic vesicle contains only one specific NT (many molecules of it)
- the idea of quantal release - synaptic vesicles aren't just same size but contain ~ same amount of NT - each neuron contains only one type of fast NT, so the synaptic vesicles in a terminal are all identical and a given neuron can provide only one type of signal |
|
|
How many classic fast NT do neurons release?
|
- only one
|
|
|
Glutamatergic neurons
|
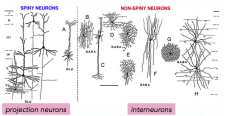
- release glutamate
- excitatory - projection neurons; spiny |
|
|
GABAergic neurons
|
- inhibitory
- release GABA - interneurons; non-spiny; making connections |
|
|
Why do neurons release only one classic NT?
|
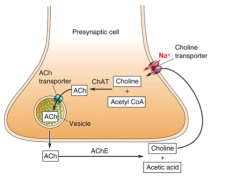
- because they synthesize and package only one NT
- synthesis occurs in cytoplasm; key enzymes catalyze synthesis - NT then transported into vesicles via NT transporters that are present in the synaptic vesicle membrane |
|
|
Glutamate synthesis
|
- from glutamine
- glial cells help out in this process |
|
|
Loading Synaptic vesicles with NT
|
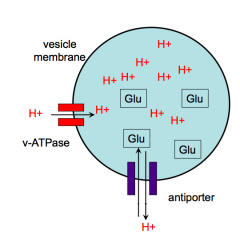
- 2 step, ATP- dependent process
- Step 1: loads protons into vesicles - Step 2: uses the energy of the proton gradient to help load glutamate or other NT - inside of vesicles is usually pretty acidic |
|
|
Small, clear synaptic vesicles
|
- can have one of this type
- small, fast NT |
|
|
Dense core granules
|
- usually contain peptide NT and biogenic amines
- slower release - can contain one of these |
|
|
Major NT's
|
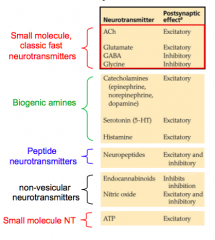
- know one of each of these at least
|
|
|
Excitatory vs Inhibitory NT's in the CNS and PNS
|
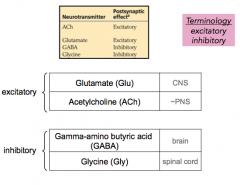
CNS = brain and spinal cord; PNS = other
GABA = brain; glycine = spinal cord - make sure you know what excitatory and inhibitory mean based on the properties of the channel |
|
|
Small, classic fast NT's
|
e.g. Glutamate, GABA, glycine, Acetylcholine
Small-molecule NT's most often mediate fast synaptic transmission |
|
|
Biogenic amine
|
e.g. catecholamines like dopamine and serotonin
- mood regulation |
|
|
Peptide neurotransmitters
|
- mediate slow cell-to-cell transmission
e.g. methionine enkephalin |
|
|
Where does synthesis of peptide NT's occur?
|
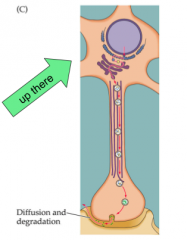
- synthesis of peptide NT's occurs in cell body
- dense core filled in cell body, then transported - Small NT's occur locally in the presynaptic terminal - enzymes transported from cell body |
|
|
Where does synthesis of small, fast NT's occur?
|
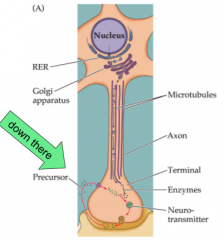
- Small NT's occur locally in the presynaptic terminal
- enzymes transported from cell body - enzymes needed for synthesis are transported to the terminal from the cell body - precursor molecules for synthesis are available throughout the cell as byproducts or are taken up from extracellular space via selective transporters - NT synthesized in cytoplasm and moved into vesicles via NT transporters |
|
|
What happens to NT once it's released?
|
- removed immediately
|
|
|
Why is NT removed immediately?
|
- if its around, the postsnaptic cell will keep firing and won't be connected to a signal releasing NT from the presynaptic cell necessarily at that moment
|
|
|
Serotonin reuptake
|
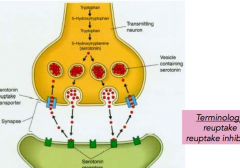
- for depression, to increase the amount of serotonin, you have a reuptake inhibitor
|
|
|
What happens when NT is in the synaptic cleft? (general)
|
- enzymatic degradation
- uptake by glia - reuptake - diffusion |
|
|
ow do astrocytes clean things up?
|
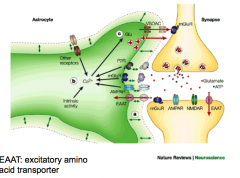
- astrocytes clean up NT and can give it back to the presynaptic neuron
|
|
|
How many inputs can the postsynaptic neuron receive?
|
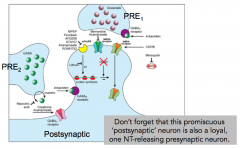
- multiple different types of input
- NOT related to what NT that it synthesizes and releases |
|
|
What dictates the types of input a synaptic neuron can receive?
|
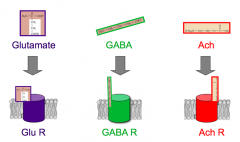
- there is a specific NT receptor for each NT
- it must express that receptor |
|
|
Ligand
|
- thing that binds a receptor (e.g. NT)
- usually the ligand binding site is on the outside of its specific receptor protein |
|
|
Ionotropic NT receptor
|
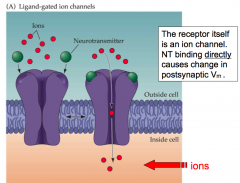
- receptor itself is a channel that opens directly when bound
|
|
|
Metabotropic receptor
|
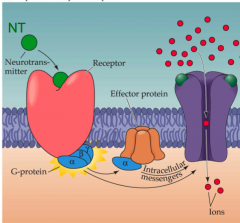
- Transmembrane G protein-coupled receptor causes a path that changes the Vm later on
- a Downstream signal transduction pathway causes change in postsynaptic Vm (usually a secondary messenger modulates nearby ion channels) |
|
|
How would you design an excitatory vs inhibitory receptor?
|
- permeability of the channel dictates whether it's excitatory or inhibitory
- ion selectivity |
|
|
What types of ion permeability do inhibitory receptors have?
|

- Chloride
|
|
|
What types of ion permeability do excitatory receptors have?
|

- opens for cations (K+, Na+, Ca2+)
- nonselective ion channel |
|
|
What is the reversal potential?
|
- for a receptor, it's analogous to the equilibrium potential for an ion channel that passes a single ion
- Reversal potential: The membrane potential at which net current through the channel is zero - often, there is permeability to multiple ions - each one is still flowing at Erev, but net current is zero |
|
|
How do you calculate reversal potential?
|

- n = # of ions that depend on the channel
|
|
|
What value dictates whether a reversal potential is excitatory or inhibitory?
|
- The reversal potential dictates whether an NT will excite or inhibit a neuron
- A reversal potential above VG Na+ threshold is excitatory - a reversal potential below VG Na+ THRESHOLD is usually inhibitory |
|
|
Are receptors much different than other ion channels?
|
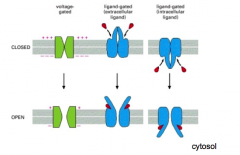
Nope. Just another one.
|
|
|
What happens to a channel when it opens and closes?
|

|
|

Discussion Question:
|
- changing the concentration can change current
- here, we'd want to hold the cell at a concentration and vary voltages - measuring reversal potential of an NT receptor - you can measure reversal potential by stimulating presynaptic input instead of applying a drug |
|
|
Does it matter whether an ion channel is ionotropic or metabotropic to determine if it's excitatory/inhibitory?
|
- NOPE. They can both be either.
|
|
|
What happens when you apply NT to cells expressing the receptor?
|
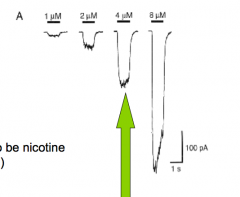
- current into or out of the cell (it just causes it)
- Focus on one concentration and apply at different holding voltages |
|
|
Measuring Reversal Potential of a NT Receptor
|
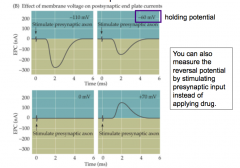
- measuring current at different holding potentials
- you can also measure the reversal potential by stimulating presynaptic input instead of applying drug (???) |
|
|
I-V curve for an AMPA Receptor (Try to draw it!)
|
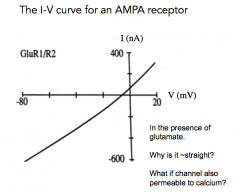
- In the presence of glutamate
- why is it ~ straight? - what if the channel was also permeable to calcium? |
|
|
What are postsynaptic potentials?
|
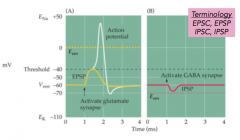
- change in the membrane potential of the postsynaptic neuron
- EPSP: excitatory postsynaptic potential - IPSP: inhibitory postsynaptic potential - EPSC/IPSC: current |
|
|
How many presynaptic neurons are there to one neuron?
|
A lot
|
|
|
What are inhibitory synapses?
|
- GABA receptors
- they're non-selective anion channels - under physiological conditions, chloride is the only relevant anion that passes through the GABA R. Consider these R's ~chloride channels |
|
|
I-V Curve for GABA R?
|
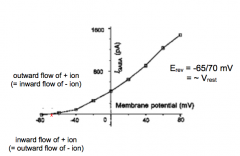
- 65 mv = Erev
- not voltage gated |
|
|
What does GABA release do to the postsynaptic cell?
|
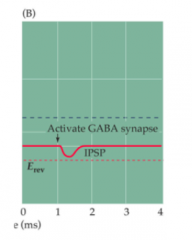
- "shunting"
- shunting inhibits the postsynaptic neuron and can be thought of in two ways: 1. Chloride current (ICl-) holds the membrane potential near rest (ECl ~= Vrest). Any simultaneous input has to compete with ICl. 2. ICl lowers overall Rm by opening many channels. By V = IR, this also makes it harder for another current to depolarize while Cl- channels open (smaller R means smaller V for same I)... |
|
|
Glutamate Receptor
|
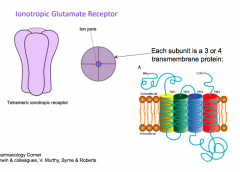
big extracellular domain for sensing ligand
- each subunit is a 3-4 transmembrane protein - all localized around the central pore |
|
|
How do ligand gated channels compare to VG channels?
|
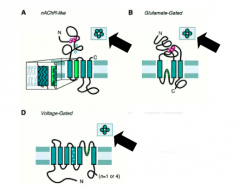
|
|
|
What are the three types of glutamate receptor?
|
- AMPA R's, Kainate R's, and NMDA R's
- although glutamate acts at all three receptors, they have different kinetics/properties |
|
|
What is desensitization?
|
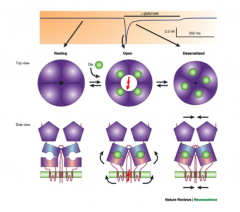
- analogous to inactivation
- still bound to the ligand, but there's no current while it's desensitized |
|
|
Whhy does desensitization occur?
|
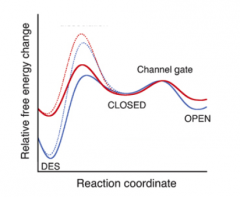
- represents a favorable energy state for some receptors
- |
|
|
What does desensitization of AMPA recpetor currents do to the glutamate response?
|

- dictates timing of glutamate response
- Why? Desensitization can allow neurons to have very fast and brief signals - Example: The time course of AMPA (Glu) R current is fast in auditory system. - have to react to differences quickly; brief signals are necessary |
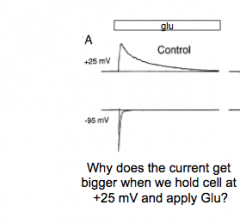
|

DQ:
|
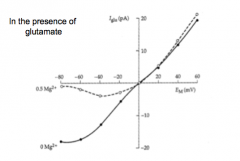
I-V curve for a glutamate receptor that is blocked by magnesium
|
|
|
What makes the NMDA receptor so special?
|
1. Blocked by MG2+ at hyperpolarized Vm. This makes the NMDA R essentially voltage dependent.
2. Calcium permeable, which is a BFD. |
|
|
Important things to consider with the NMDA receptor
|

|
|
|
What are the implications of the Mg+ blockage for the function of the glutamate receptor?
|
1. blocked by Mg2+ at hyperpolarized Vm
2. Makes NMDA R essentially voltage dependent 3. Ca2+ permeable: big deal. Triggers strengthening of synapses. 4. A Coincidence detector: 2 things need to happen simultaneously: 1. NT and 2. Depolarization |
|
|
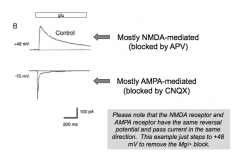
NMDA R current vs AMPA R current
|
|
|
|
What happens when you add AP5 (blocks NMDA R's)?
|

- Voltage-sensitive component is not affected by CNQX. However, the fast, voltage insensitive component is lost with CNQX block of AMPA R's.
|
|
|
Kinetics of AMPA vs NMDA
|
|
|
|
Do NMDA receptors desensitize as well?
|
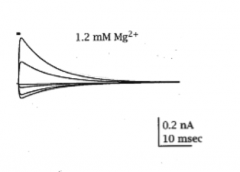
|
|
|
How would you tell the difference between an AMPA vs. NMDA current?
|
- timing and whether or not it's open at certain voltages
- pharmacology - desensitization |
|
|
Silent synapse
|
- NMDA
- won't fire if NT is around but it's not depolarized - needs to have depolarization and NT (e.g. concurrent firing of an AMPA receptor) |
|
|
Silent synapse e.g.
|
- See notes
- if 1 and 2 fire at the same time, you'll see an NMDA response because AMPA enters the cell - If 1 and 2 are coupled, the "silent synapse" will be loud - 2 inputs can have a different impact depending on timing |
|
|
GABA recpetor
|
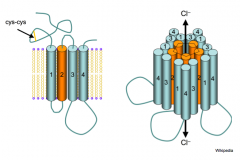
- structure is nAChR-like (5 subunits)
- T2 lines the pore - Flow through GABA channels = stronger inhibition of postsynaptic cell |
|
|
What types of molecules modulate GABA receptors and how do they modulate it?
|
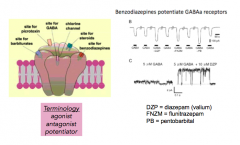
- NOTE: if you put any of the drugs by itself, there would be nothing
- need the NT and the drug present to see an effect |
|
|
WhAgonist
|
- opens the channel
|
|
|
Antagonist
|
- closes the channel
|
|
|
Potentiator
|
enhances sensitization...?
|
|
|
How can you use pharmacology to distinguish between GABA receptors and glycine receptors?
|
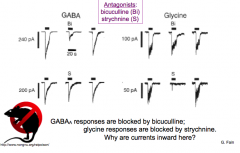
- THM: Bicuculline blocks GABA (brain) and strychnine blocks glycine (S)
- WHY ARE CURRENTS INWARD? |
|
|
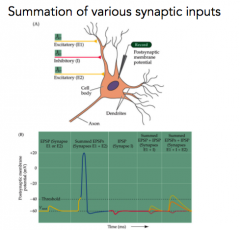
Summation of inputs?
|
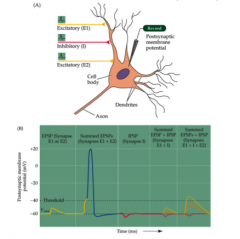
- To increase a postsynaptic response, stimulate it twice (temporal stimulation)
- OR, stimulate two at the same time (spatial) - because GABA is so close to Vrest, it sometimes doesn't look like anything |
|
PRACTICE the questions we did and class
|
example questions:
1. When it's on, a single step occurs 2. Don't take a step 3. Take a step only if something is happening at the same time 4. BIG step if a puddle is present (more NT on step neuron) 5. Same, but if a puddle is present, don't take a step |
|
|
What happens to proteins over time? Why?
KEY POINT |
- they change over time
- this: 1. changes synaptic strength and 2. changes behavior |
|
|
How could you study how synapses change over time?
|
- look at morphology
- look at an organism that learns and tracks changes over time (Aplysia!) |
|
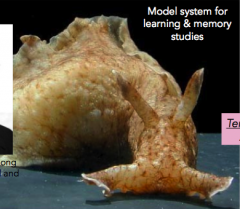
What is this thing and how do you study it?
|
-it's Aplysia californica, or the California seaslug
- Erica Kandel studied it and won the Nobel prize in 2000 - cellular basis of memory and learning |
|
|
Aplysia gill withdrawal
Design a cellular circuit that would achieve this behavioral response (simple as possible) |
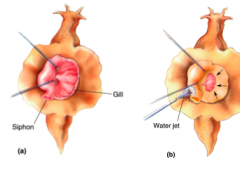
- gill withdraws when the siphon is stimulated
|
|
|
Design a cellular circuit that would achieve this behavioral response of gill withdrawal (simple as possible)
|
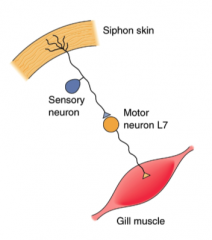
|
|
|
Habituation
|
- following repeated stimulation over time, gill withdrawal habituates or ceases to respond to stimulus
|
|
|
How could you test if the cellular mechanism for the Aplysia withdrawal habituation is presynaptic or postsynaptic?
|
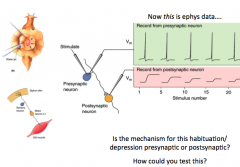
- record from both
- quantal analysis (mini analysis) shows that release probability goes down during habituation (fewer vesicles released) --> PRESYNAPTIC |
|
|
What is the cellular mechanism of habituation for Aplysia?
|
- decreased efficacy of presynaptic VG Calcium Channels
- less calcium = less vesicle release = less NT = lower postsynaptic response |
|
|
What is the mechanism for resensitizing the gill withdrawal reflex when you touch the tail?
- normally, the tail doesn't cause a reflex |
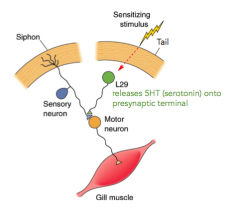
- releases 5HT(serotonin) onto presynaptic terminal
- closes K+ channels - lengthens AP and allows more Ca2+ into the presynaptic terminal |
|
|
What does 5HT receptor activation do, more specifically?
|
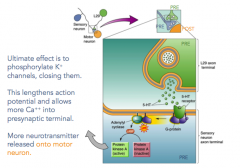
- more neurotransmitter then released onto the motor neuron
|
|
|
What happens when you block K+ channels in the presynaptic terminal?
|
- it lengths the AP
|
|
|
Sensitization
|
- the opposite of habituation
- when you make a reflex sensitive to a certain process again? |
|
|
What are some general short term types of synaptic modification?
|
Depression (habituation, short term depression)
Strengthening (sensitization, facilitation) |
|
|
What are some general long term types of synaptic modification?
|
- long term potentiation (LTP)
- long term depression (LTD) |
|
|
What is the length of synaptic delay?
|
- 1/2 msec - 1 msec
|
|
|
What is the general spacing of the synapse?
|
e.g. synaptic vesicle is about 35 nm
synaptic cleft is ~ 20nm membrane is ~5nm |

