![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
268 Cards in this Set
- Front
- Back
|
Forebrain
|
Telencephalon + Diencephalon
|
|
|
Mesencephalon
|
Midbrain
|
|
|
Metencephalon
|
Pons + cerebellum
|
|
|
Myelencephalon
|
Medulla
|
|
|
Rhombencephalon
|
Metecephalon + Myelencephalon (Pons, Cerebellum, Medulla)
|
|
|
Brainstem
|
midbrain, pons, medulla
|
|
|
Brain
|
Brainstem, cerebellum, forebrain
|
|
|
White matter
|
myelinated axons (cortex inwards, spinal cord outwards)
|
|
|
Grey matter
|
cell bodies (cortex outwards, spinal cord inwards)
|
|
|
Association (White matter)
|
Mediates communication between areas WITHIN a hemisphere
|
|
|
Commissural (white matter)
|
Mediates communication between areas BETWEEN hemispheres
Major Commissural = Corpus Collosum |
|
|
Projection (white matter)
|
Mediates communication between cortical and subcortical sites
Major Projection - Internal capsule |
|
|
Lateral Geniculate Nucleus
Location? Function? |
Lateral Geniculate Nucleus
Location: Thalamus (Diencephalon) Function: relay station between retina and primary visual cortex |
|
|
Suprachiasmatic nucleus
Location? Function? |
Suprachiasmatic nucleus
Location: hypothalamus (Diencephalon) Function: trigger circadian clock |
|
|
Pretectal Nucleus
Location? Function? |
Pretectal Nucleus
Location: Midbrain (Mesencephalon) Function: afferent limb of pupillary light reflex |
|
|
Superior Colliculus
Location? Function? |
Superior Colliculus
Location: Midbrain (Mesencephalon) Function: Contribute to Eye Movements |
|
|
Magnocellular
|
- Dorsal (parietal pathway)
- Depth and Motion (location & movement) - 1st 2 layers - detects fast moving stimulus |
|
|
Parvocellular
|
- Ventral (temporal pathway)
- Form & Color (Color vision & acuity) - projects deeper into the primary visual cortex -layers 3-6 |
|
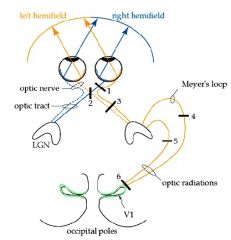
What portion of the visual field is lost at lesion 1?
|
Monocular Blindness
Lesion: Optic Nerve total blindness in 1 eye |
|
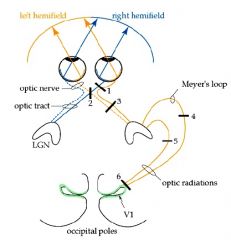
What portion of the visual field is lost at lesion 2?
|
Lesion: Optic Chiasm
Bitemporal hemianopia Cut Nasal Retina Input. |
|
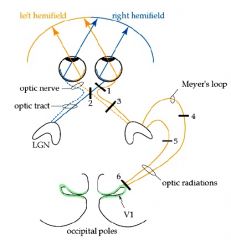
What would be lost on the visual field in lesion 4?
|
Superior Homonymous Quandrantanopia
|
|

What part of the visual field would be lost at lesion 7?
|
Macular Sparing
Fovea Spared. notched hemifield |
|
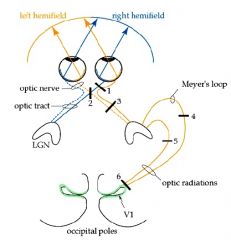
Where part of the visual field is lost in lesion 3?
|
Homonymous hemianopia
Lesion in Optic tract or Posterior cerebral artery dysfunction |
|
|
Inferior colliculus
Location? Function? |
Inferior colliculus
Location: Midbrain (Mesencephalon) Function: involved in the localization of sound |
|
|
Type of receptor on ON bipolar cells
|
E (Glutamate [metabotropic receptor])
|
|
|
Superior Visual Field
is temporal/parietal radiation? is above/below calcarine sulcus? |
Superior Visual Field
is temporal radiation is below calcarine sulcus |
|
|
Inferior Visual Field
is temporal/parietal radiation? is above/below calcarine sulcus? |
Inferior Visual Field
parietal radiation above calcarine sulcus |
|
|
Ocular Dominance Columns
|
Alternating by which eye input comes from [ipsilateral & contralateral]
|
|
|
Orientation Columns
|
Light Orientation (horizontal, vertical)
|
|
|
Dark Current
|
- photoreceptors are depolarized and releasing glutamate in the dark.
|
|
|
What is the neurotransmitter for photoreceptors?
|
glutamate
|
|
|
Protanopia
|
Red cone color blindness
|
|
|
Deuteranopia
|
Green Cone Color Blindness
|
|
|
What is the rarest type of color blindness?
|
Blue
|
|
|
What kind of receptor is a ON Bipolar cell?
|
Glutamate (metabotropic)
|
|
|
What kind of receptor is a OFF Bipolar cell?
|
Ionotropic
|
|
|
Epidural Hematoma
|
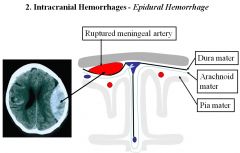
- Hemorrhage of ARTERY (usually Middle Meningeal)
- Biconvex shape - Lucid for a while and then die suddenly |
|
|
Subdural Hematoma
|
- Hemorrhage of bridging VEIN
- Crescent Shaped - Venous System - lower pressure than arterial. = takes approx 4-6 weeks for symptoms. - Vague Symptoms. - Chronic rather than acute - Trauma or Elderly |
|
|
emx
|
homeobox gene
- deficiency = schizencephaly (split cortex) |
|
|
otx
|
homeobox gene
- deficiency = epilepsy |
|
|
hox genes
|
direct fate of cells within a segement
- ie. master gene in drosophilia |
|
|
BMP (Bone morphogenic Protein)
|
- induction of neural plate
- Early - suppress neural differentiation and promotes epidermal tissue - Later - is blocked so that ectoderm can form neural plate |
|
|
PMP22
|
mutation of PMP22 = Charcot Marie Tooth Syndrome
-PMP22 =produced in schwann cells for myelination |
|
|
Sonic Hedgehog
|
- NOT a homeobox gene
- influences development of serotonergic neurons - hindbrain dopaminergic neurons - posterior midbrain oculomotor neurons - anterior midbrain - secreted by notochord - acts on floor plate to induce netrin formation is a protein |
|
|
Hensen's Node
|
Anterior-Posterior Hox gene expression
|
|
|
Radial Glia persist in adults as
|
Muller cells (Retina)
Bergman Glia (cerebellum) |
|
|
astrotactin
|
proteins that mediate neuronal migration
if blocked = no cell migration Receptor = Integrin |
|
|
Reeler
|
large extracellular matrix glycoprotein
If Mutated - neurons fail to migrate past each other - positions inverted - Expressed in Cajal-Retzius cells - tell cells to 'get off' the glial monorail |
|
|
Kallman's Syndrome
|
- no sense of smell
- no sexual maturation |
|
|
PNS neural crest cells migrate along pathways that
|
LACK
1) axons tracts 2) organized glial structure |
|
|
laminin
|
- made by Schwann cells after injury
- promotes neural outgrowth Antibody against laminin = block neurite extension and outgrowth |
|
|
What activates transcription of Hox genes?
|
Retionic Acid
- creates a Anterior- posterior gradient |
|
|
What kind of neurons can move without radial glial cells?
|
neurons with Gonadotropin-releasing hormone
failure of GnRH migration = Kallman Syndrome = ↓ olfactory and sterile |
|
|
Axon growth is influenced by:
|
Diffusible Molecules and Proteins from Extracellular Matrix
1. Pioneer Axon - leads the way. has extensive filopodia 2. Netrin - released by floor plate. causes axons to cross anterior commissure 3. Laminin - secreted by Schwann cells after accident; promotes neural outgrowth 4. Growth cone - enlarged axon extension. Receptors are on axon surface. cones may release enzymes to clear pathway and change substrate |
|
|
Growth cone
|
enlarged axon extension. Receptors are on axon surface. cones may release enzymes to clear pathway and change substrate
- in axonal growth |
|
|
Netrin
|
released by floor plate. causes axons to cross anterior commissure
- in axonal growth |
|
|
Pioneer Axon
|
leads the way. has extensive filopodia
- in axonal growth |
|
|
Leukemia Inhibitory Factor
|
- peptide secreted to change crest cells
- induces differentiation in immune system |
|
|
agrin
|
released by nerve.
makes receptors on NMJ concentrate on end plate |
|
|
semaphorin
|
short range inhibitory signal
|
|
|
Trembler mouse
|
- myelin formed to fail
- genetic defect in Schwann cells Sciatic nerve tansplant experiment |
|
|
Charcot Marie Tooth Diease
|
PMP22 mutation
gly to asp (amino acid substitution - peripheral myelin fails to form properly |
|
|
NCAM
|
Neural Cell Adhesion Molecule
- binds to itself and causes axons to stick to one another - bound axons are wrapped by shared glia -Fab part of antibody can disrupt bundling of growing axons |
|
|
Nerve Growth Factor
|
- from snake venom and salivary glands
- causes growth of neurites - required for sensory cell outgrowth - antiNGF = smaller Dorsal Root Ganglion - affects Sympathetic NS but not Parasympathetic - each cell types CRITICAL PERIOD = survival depends on supply of NGF - Transported into Soma, regulates synthesis of NORE via 1 tyrosine hydroxlase, dopamine b-hydroxylase |
|
|
Brain derived neurotropic factor (BDNF)
|
- protein in CNS that promotes survival of DRG
- very similar to NGF - can rescue cells from neuronal death if administered |
|
|
Neurotrophin
|
- Family of growth factors, including BDNF and NGF
- dimers with basic regions held together by cysteine residues In Early development, before sensory neurons innervate target, Neurotrophin needed for: - neural crest cells -sensory neurons -Dorsal Root Ganglion -Trigeminal Ganglia |
|
|
p75
|
low affinity, fast NGF receptor
found on neurons and non-neuronal cells |
|
|
p140prototrk (trk)
|
high affinity receptor
- found only in neurons - mediate cell survival and neurite outgrowth - intracellular domain has Tyrosine Kinase - activates Intracellular Signalling Pathways: 1. PLC 2. Phosphatidylinositol 3 Kinase 3. MAP Kinase |
|
|
trkA
|
High affinity receptor for NGF
|
|
|
trkB
|
High affinity receptor for BDNT and NT-4/5
|
|
|
trkC
|
High affinity receptor fro NT-3
|
|
|
BDNF influences
|
retinal ganglion cell branching and remodeling
dendritic growth of cortical neurons formation of ocular dominance columns within developing visual cortex |
|
|
Ephrin
|
- responsible for repulsive interactions during cell signaling
- cell migration, axon pathfinding and cell intermingling - part of receptor tyrosine kinases ephrin-A2 and ephrin A-5 are in tectum |
|
|
Thalidomide
|
- reduce limbs to flipper like appendages
1. primary effect = neurons 2. secondary effect = bone growth |
|
|
Albinism
|
frequent miswiring of retinogeniculate connections
|
|
|
Microglia
|
macrophage of CNS
|
|
|
Astrocytes
|
- homeostatis at synapse
- regulates chemicals - recycles Neurotransmitter - building block of BBB - Release antioxidants to protect neurons - uptake of excess Neurotransmitters via transporters - secretes cholesterol for neural development Signals via IP3/Ca2+ |
|
|
oligodendrocytes
|
coats CNS with myelin
|
|
|
Ependymal Cells
|
Secretes CSF
|
|
|
Radial Glia
|
- Scaffold that developing neurons climb on
Persists in the adult as: - Muller cells - retina - bidirectional communication with neurons - Bergmann Glia - cerebellum - regulate synaptic plasticity |
|
|
Bergmann Glia
|
Radial Glial cells in the cerebellum - regulate synaptic plasticity
|
|
|
Muller cells
|
Radial glia in retina - bidirectional communication with neurons
|
|
|
Schwann Cells
|
myelinate PNS axons
phagocytic activity |
|
|
Satelitte cells
|
regulates external chemical environment.
- surround sensory, sympathetic, parasympathetic |
|
|
Tanycytes
|
ependymal cells in 3rd ventricle. Bipolar cells that link CSF to neuroendrocrine events
|
|
|
Enteric Glial
|
analagous to CNS astrocyte but in Enteric Nervous system
|
|
|
Tripartite Synapse
|
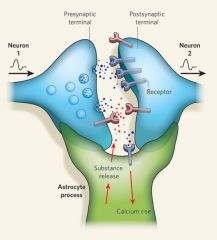
close proximity of pre, post and glial cell
- both neuron and glia can release neurotropic factors - Glial-cell derived neurotropic factor (GDNF) - Brain derived Growth Factor (BDNF) - Neurotropin-3 |
|
|
Perivascular Astrocytes
|
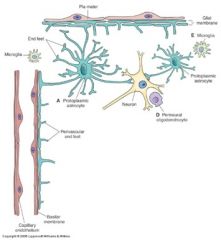
astrocytes that maintain blood flow and osmotic balance in brain
|
|
|
Tanycyte
|
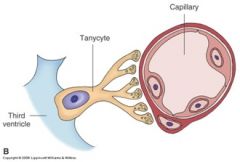
bipolar cells that link CSF to neuroendocrine events.
- radial glial that differentiate with astrocyte like properties |
|
|
Schwann cells
|
in PNS
- myelinate AND phagocytic activity |
|
|
glial scar
|
- During trauma or ischemia
- astrocyte form scar to isolate neurons from each other - presence of glial scar = limits the possibility of axonal regeneration in CNS |
|
|
Astrocytes store _____________
|
glycogen
- can be converted to lactate and be used as an alternate supply of ATP |
|
|
Astrocytes have what kind of receptors:
1. Ionotropic 2. Metabotropic |
both
|
|
|
Spatial Buffering/Potassium Siphoning
|
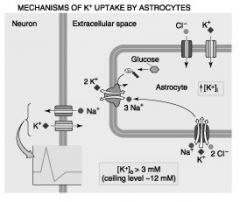
- astrocytes take in K+ from extracellular fluid and redistributes to its neighbors (via gap junction)
- K+ from action potentails |
|
|
Amyotrophic lateral sclerosis
|
loss of brain and spinal cord motor neurons
- mutation in Superoxide Dismutase and astrocytes |
|
|
Gliosis
|
hyperplasia and hypertrophy of astrocytes in response to injury in CNS
= Glial Scar |
|
|
Glial Fibrillary Acidic Protein (GFAP)
|
- encodes filament protein for mature astrocytes
Disease = Alexander Disease |
|
|
Anterograde Degeneration
|
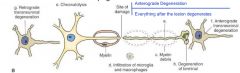
degeneration of the neuron postsynaptic to the damaged neuron
- Wallerian degeneration - after the axon has been cut |
|
|
Retrograde Degeneration
|
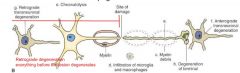
degeneration of the neuron, before the lesion
|
|
|
Chromatolysis
|
when lesion next to cell body
- swelling and movement of cell organelles away from the cell body |
|
|
Multipolar neurons are
Efferent/Afferent |
Efferent
|
|
|
Pseudounipolar neurons are:
|
Afferent
|
|
|
Bipolar cells are:
Motor/Sensory |
Sensory
|
|
|
synaptic potentials
1. do not spread with decrement 2. spread with decrement |
spread with decrement
|
|
|
action potentials
1. do not spread with decrement 2. spread with decrement |
do not spread with decrement
|
|
|
Collateral Axon branch
|
branch of main axon that feeds back onto soma providing modulation of cell firing
|
|
|
Nissl body
|
histological sign of rough ER; site of protein synthesis
|
|
|
What is the main determinant of shape of the neuron?
|
cytoskeleton
ie. microtubules, neurofilaments, microfilaments" |
|
|
Multiple Sclerosis
|
- degeneration of myelin in CNS
- autoimmune - reduced axonal velocity - CN II is only cranial nerve demyelinated in MS as well as problems with hearing and balance (caused in brainstem, not CN) |
|
|
Guillain-Barr Syndrome
|
-degeneration of myelin in PNS
- affects both myelin and axons themselves |
|
|
Neurofilament
|
-most common filament in neurons
-highly stable -undergo little turnover - Scaffolding for cytoskeleton of neuron |
|
|
Microtubule
|
-13 protofilaments forming a tubule (α/β)
- associated with dynein and kinesin - damage to microtubule = cell death - transport between nerve cell and soma" |
|
|
Remak Fiber
|
unmyelinating Schwann cells that envelope small axons to regulate [K+]
|
|
|
Choroid Plexus
|
Ependymal cells - Group 1
Secretory, grouped by TIGHT JUNCTIONS |
|
|
Ventricles & Central Canal
|
Ependymal cells - group 2
- Assists in CNS CSF circulation joined by GAP JUNCTIONS - solutes pass paracellularly |
|
|
Resting potential of neuron
|
-65
|
|
|
Resting potential of skeletal muscle
|
-90
|
|
|
Resting membrane potential of photoreceptor
|
-40
|
|
|
In neurons, membrane potentials are affected by which ion?
K+ Ca2+ Na+ H+ |
K+
|
|
|
The main difference between neurons and glia is:
|
The main difference between neurons and glia is how they transfer info
Neurons: Action Potentials Glial cells - Intra/Inter-cellular Ca2+ signalling |
|
|
Dynein
|
"- fast RETROGRADE transport
- nerve ending back to soma - motor protein for movement in cilia and flagella - inactivated in soma and carried back to nerve ending -ATPase - carries lysozymes, enzymes and recycled vesicle membranes" |
|
|
Kinesin
|
fast ANTEROGRADE transport
- soma to nerve ending - ATPase - allows attachment of large structures (vesicles, mitochondra) - Kinesin is inactivated at nerve ending and carried back via retrograde" |
|
|
What excites astrocytes?
|
[Ca2+] not voltage
|
|
|
Which is faster: electrical signaling or axonal transport?
|
Electrical Signaling
|
|
|
Channelopathies
|
dysfunctional ion channels
|
|
|
Cytotoxic Edema
|
edema following ischemia (interrupted blood supply)
- affects Grey more than white - edema b/c failure to supply Na+/K+ pump with ATP = ions dissipate - Increased Intracellular water and Na+ in edema fluid - Clinical Disorders: Hypoxia, Water intoxication, Ischemia |
|
|
Vasogenic Edema
|
blood vessels become permeable and fluid accumulates in extracellular space
|
|
|
The Membrane Potential (Vm) depends on
|
number of open channels for ions
conductances of the open ion channels equilibrium potentials for those ions |
|
|
What happens with lethal injection?
|
- Lethal Injection = KCl
-raise extracellular K+ - depolarizes excitable cells - fails to generate another action potential b/c voltage gated Na+ channels are inactivated |
|
|
Efferent neurons and Interneurons
|
have membrane proteins that are chemoreceptors for NT
|
|
|
Afferent
|
membrane receptors responding to a specific type of stimulus
|
|
|
Golgi Tendon Organ
|
Mechanoreceptor in muscle
- measure TENSION - muscle mechanoreceptor measures stretch |
|
|
Mechanoreceptors in muscle
|
measure STRETCH
- golgi tendon organs measure TENSION |
|
|
anosmia
|
loss of olfactory sensation
|
|
|
spasticity
|
increase of muscle tone.
hyperexcitability of stretch reflex with decrease in reflex threshold |
|
|
Enkephalin
|
regulates nociception
made in hindbrain opoid pepetide |
|
|
Biogenic amines
|
catecholamines, indoleamine, imidazoleamine, purine
|
|
|
Neuropeptide Y
|
- in CNS and PNS
- CNS - cortical excitability, circadian rhythm, stress response, pain processing, emotion, food response - PNS - cardiovascular function, blood pressure, vasoconstriction |
|
|
Vasopressin
|
from hypothalamus
-stored in posterior pituitary Vasopressin = ADH |
|
|
Dynorphin
|
regulates nociception
opoid Leu-Enkephalin |
|
|
Nitric Oxide
|
not stored in vesicles
made on demand release not Ca2+ dependent doesn't interact with postsynaptic cleft |
|
|
Synapsin
|
Gets phosphorylated. release vesicles from cytoskeleton
|
|
|
Rab Protein
|
transports vesicle to site of exocytosis
(moves vesicle to active site) |
|
|
Synaptobrevin
|
Docking of vesicle of nerve membrane.
(partially docked) - Binds to syntaxin. |
|
|
Synptotagmin
|
Docking of vesicle of nerve membrane.
(fully docked) - Binds to neurexin. |
|
|
Synaptotophyin
|
forms fusion of vesicular and nerve membrane.
Fully docked to nerve membrane |
|
|
small clear vesicles
|
- AcH, serotonin, glycine
- recycled locally (at the synapse) - low nerve stimulation (10Hz) causes a rise in Ca2+ near active zone and causes exocytosis |
|
|
dense core vesicles
|
- peptides (NE, Serotonin)
- high nerve stimulation rate (100Hz) cause a rise in Ca2+ near active zone = exocytosis |
|
|
Botulinum Toxin
|
- blocks Ach release
- blocks synaptobrevin (Ach can't dock) - diplopia, dysphasia, dysphagia dry mouth = botox injection |
|
|
Tetanus Toxin
|
blocks glycine release
- blocks synaptobrevin (glycine can;t dock) - |
|
|
α-Latrotoxin
|
massive release of AcH = depletes all of Ach
then nothing. flaccid paralysis |
|
|
4-aminopyridine (4-AP)
|
- blocks K+ channels and increases duration that Ca2+ release = more AcH
|
|
|
neomycine/streptomycin
|
inhibit Ach exocytosis at high concentration = blocks nACHr
|
|
|
Monoamine Oxidase breaks down:
|
norepinephrine, epinephrine, dopamine, serotonin
|
|
|
Catechol O-methyltransferase (COMT) degrades:
|
norepinephrine, epinephrine, dopamine,
Does not degrade serotonin (unlike MAO) |
|
|
M2 Receptor
|
Ach receptor
In Heart ↓ cGAMP inhibits pacemaker cells coupled to K+ and Ca2+ Channels = closure of Ca2+ channels Inhibitory - slower spontaneous depolarization and slower HR |
|
|
M3 receptor
|
Ach Receptors
- stimulates exocrine glands and eccrine glands - also stimulates smooth muscle ↑ PLC = ↑ IP3 and DAG ↑ Ca2+ release |
|
|
Excitatory M receptors
|
M1, M3, M5
via phospholipase M1, M3 = brain M3 - secretory and smooth muscle |
|
|
Inhibitory M receptors
|
M2, M4
M2 = heart |
|
|
Dopamine
|
mostly in CNS. not much in PNS
|
|
|
Norepinephrine
|
activates adrenoceptors of smooth muscle, glands, cardiac
(Sympathetic) |
|
|
Ephinephrine
|
released by chromaffin cells
enters circulation ultimate activates adrenoceptors |
|
|
Isoprenaline
|
synthetic catecholamine
similar structure to ephinephrine |
|
|
D1 and D5 Dopamine Receptors
|
↑ cAMP production
|
|
|
D2 (D3, D4) Dopamine receptors
|
↓ cAMP production
↑ D2 = psychosis - give D2 anatagonist ↓D2 = parkinsons. - give D2 agonist |
|
|
Glycine
|
inhibitory in the spinal cord
- ionotropic - permeable to Cl- - 3 glycines to bind - resembles nACHr |
|
|
GABA
|
- inhibitory in the brain and brainstem
- 2 GABA to bind - mediates FAST IPSP -metabotropic - indirect K+ channels open thru 2nd messenger - mediate slow inhibition of postsynpatic neuron -anti-anxiety drugs inhibits Amygdala (responsible for fear/anxiety) |
|
|
Glutamate
|
has both metabotropic and ionotropic
|
|
|
AMPA
|
allows Na+ to enter
Ionotropic |
|
|
NMDA
|
allows Ca2+ in
(blocked by Mg2+) - when v. large depolarization, many EPSP = membrane induces Mg2+ to leave Unblocked NMDA = Ca2+ enters = - long lasting physiological changes in cell - activated during intense synpatic activity |
|
|
SSRI
|
for clinical depression
- allows more available SEROTONIN in CNS - slows down 5-HT receptor = ↓ reuptake |
|
|
Pilocarpine
|
naturally occurring alkaloid
mimics AcH - agonist at all muscarinic receptors - used for glaucoma (faciliates fluid drainage from eye via canal of schlem) |
|
|
Beta blockers
|
antagonist of B1 receptors
treat hypertension - antagonize vasoconstriction action of Norepinephrine |
|
|
how does the composition of CSF compare to serum (in terms of Protein, K+, Na+, Ca2+ and Cl-)?
|
CSF
- less protein - less cations (K+, Na+, Ca2+) - more Cl- - produced by direct secretion |
|
|
Presence of RBC and high [protein] in CSF is a sign of
|
Subarachnoid hemmorhage
|
|
|
Glucose crosses the BBB via
|
GLUT1 transporter.
facilitated diffusion |
|
|
L-dopa crosses the BBB via
|
facilitate diffusion
|
|
|
Glycine crosses BBB via
(From brain to blood) |
Na+ dependent secondary active transport
|
|
|
CSF is absorbed by
|
arachnoid villi in the superior sagittal sinus
|
|
|
Presence of ↑[cell] and ↓ [protein] in CSF is a sign of
|
Bacterial meningitis
|
|
|
Presence of ↑[cell] and normal [protein] in CSF is a sign of
|
Viral Meningitis or brain tumor
|
|
|
Papilloma
|
tumor in CSF
- communicating hydrocephalus - all ventricles enlarged |
|
|
Normal Pressure hydrocephalus
|
- no rise in intracranial pressure
- reduction of brain volume - usually in elderly only - enlarged ventricles, sulci and fissures with flattening cortical gyri against skull |
|
|
ependymal cells transfer water and nutrients via
paracellular/transcellular |
paracellular
|
|
|
Dandy Walker Syndrome
|
Foramina of Luschka and Magendie fail to develop
- Dilatation of all 4 ventricles - congenital |
|
|
Congenital Hydrocephalus
|
Usually due to blockage of cerebral aqueduct
|
|
|
How is Intracranial Pressure measured?
|
Spinal tap (L4/L5 or L3/L4)
|
|
|
1A afferents
|
- excite motor neurons & interneurons
in spinal cord - release glutamate |
|
|
EPSP
metabotropic or ionotropic |
Ionotropic - CATION receptors
|
|
|
IPSP is invoked by which neurotransmitter?
|
Glycine or GABA
|
|
|
Renshaw Cell
|
Interneuron
signals from a motor neuron running collaterally back via the ventral horn into the spinal cord, there were interneuron’s firing with a high frequency, resulting in inhibition |
|
|
Transient Ischemic Attack
|
acute loss of cerebral functions for ~24 hours
-caused by temporary blockage of cerebral circulation -full recovery likely. |
|
|
Reversible ischemic attack
|
neurological deficit is acute loss of cerebral or monocular function
-symptoms last longer than 24 hours; -recovery is still likely. |
|
|
Striae medullares
|
strands of the vestibulocochlear nerve (CN VIII) which wind around inferior peduncle, disappearing into median sulcus.
|
|
|
Gracile tubercle
|
function is for fine touch and proprioception.
corresponds to neurons of gracile nucleus which is one of dorsal column nuclei |
|
|
Cuneate tubercle
|
Cuneate nucleus is part of dorsal column-medial lemniscus system, which carries fine touch and proprioception information to thalamus and cerebellum.
- located inferior to gracile tubercle. |
|
|
Blood supply of the Pons
|
branches of the Basilar artery
|
|
|
locked-in syndrome
|
- not enough blood supply (i.e. due to stroke) to ventral portion of pons
-horizontal eye movement and facial expression (along with all other voluntary muscle actions) will be non-functional |
|
|
Ataxia
|
problem in performing smooth, coordinated muscle movements, generally resulting in a wide gait.
-Ataxia is caused by a problem with cerebellum or cerebellar peduncles. |
|
|
Erlanger & Gasser
|
- used for spinal nerves, and can be both motor and sensory.
- A, B,C,D - A = thickest and highest conduction velocity |
|
|
Lloyd classification
|
- used for afferent nerves in skeletal muscle
- I-IV I = thickest and highest conduction velocity IV = thinnest. slowest |
|
|
Membrane Conductance (equation)
|
membrane conductance = total # of open channels x conductance of each channel
|
|
|
Tetrodotoxin
|
blocks Na+ entry
|
|
|
Saxitoxin
|
blocks Na+ Entry
|
|
|
4-aminopyridine (4AP)
|
selectively block K+ channels = will prolong action potential.
- used for people with muscular weakness or disease at the NMJ |
|
|
Anesthetics
|
block Na+ channels on pain receptors
|
|
|
safety factor
|
every excitatory signal is ~5x as strong as is needs to be. therefore it can still move thru an demyelinated area
|
|
|
Synaptic delay
|
occurs between arrival of impulse at synaptic bouton (nerve ending) and response of postsynaptic cell;
related to speed of diffusion, but mostly affected by transmitter release (exocytosis). - Shorter in Ionotropic. Longer in Metabotropic |
|
|
Which Neurotransmitter DIFFUSES out of synaptic cleft?
|
neuropeptides
|
|
|
Strychnine
|
blocks glycine receptors in CNS - no inhibitory signals = muscle spasm and inference with breathing
|
|
|
Morphine
|
binds to receptors of neuropeptide on C fibers (pain fibers) located in substantia gelatinosa
|
|
|
Cocaine
|
blocks reuptake of dopamine, serotonin, norephinephrine = prolonged activity
prolonged stimulation = down regulation of receptors |
|
|
Botulinum
|
inhibits exocytosis by inhibiting docking of synaptic vesicles.
|
|
|
Lambert-Eaton syndrome
|
auto-immune disease characterized by muscle weakness due to attack on Ca2+ channels in motor nerve terminals.
|
|
|
Neostigmine
|
inhibits AchE in synaptic cleft allowing Ach concentration to remain high.
|
|
|
Myasthenia gravis
|
is auto-immune disease where anti-bodies recognize nAchR, reducing their number on end plate, resulting in condition where there is not enough cation channels to reach threshold.
- treated with neostigmine (also give atropine because achE is not good for Cardiovascular) |
|
|
Merkel's disk
|
receptor for touch discrimination
|
|
|
Ruffini's Endings
|
receptor for skin stretch
|
|
|
Meissner's corpuscle
|
receptor for vibration
- highest sensitivity - low frequency |
|
|
Pacinian Corpuscle
|
receptor for vibration
- high frequency - low sensitivity |
|
|
Factors Contributing to High Spatial Resolution
|
1. High density of cutaneous mechanoreceptors
2. Small receptive field 3. Large cortical area involved 4. Special mechanisms, ie. lateral inhibition |
|
|
stereognosia
|
ability to perceive properties of a coherent object, like its size, shape, texture, by holding it the hand
|
|
|
The first sense lost in peripheral neuropathies is usually:
|
vibration
|
|
|
Tabes Dorsalis
|
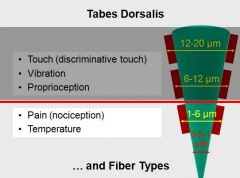
- Destruction of DRG cells with large diameter myelinated axons
- Consequence of Syphilitic infection - severe deficit in touch and proprioception (large diameter myelinated axons) - Nociception and temperature remain unaffected |
|
|
Phantom limbs is due to:
|
reorganization of the cortical maps
ie. touching the face induced sensations that were perceived to have originated from amputated hand - facial region takes over 'unused' cortex regions |
|
|
Nociceptive Pain
|
from stimuli that have the potential to cause tissue damage
|
|
|
Neuropathic Pain
|
pain sensation from aberrant somatosensory processing in the PNS or CNS
|
|
|
First Pain vs. Second Pain
|
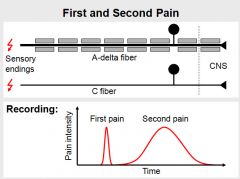
First Pain
- myelinated Aδ fibers (faster) - sharp, pricking sensation - activation of thermal or mechanical nociceptors Second Pain - unmyelinated C fibers (slower) - burning sensation - activation of polymodal nociceptors |
|
|
Visceral Pain is carried on what type of fiber?
|
unmyelinated C fibers
- burning sensation |
|
|
What activates three chemicals activate nociceptors?
|
1. K+ from damaged cells
(intracellular K+ leaks into extracellular. will depolarize surrounding cells) 2. Bradykinin from blood 3. Histamine from mast cells |
|
|
What 3 chemicals cause hyperalgesia?
|
Hyperalgesia = increased sensitivity to pain after nociceptors have been activated
Leukotrienes - from damaged cells Prostaglandin - from damaged cells Substance P - from primary afferents |
|
|
What are the effects of opioids?
|
- ↓ duration of action potential from afferent neurons
- ↓ amplitude of excitatory post synaptic potential - hyperpolarize 2nd order neuron in pain pathway |
|
|
How does aspirin regulate pain?
|
It inhibits cyclo-oxygenase
cyclo-oxygenase is the enzyme responsible for the synthesis of Prostaglandins. - Prostaglandins increase pain sensitivity |
|
|
Dorsal rhizotomy
|
- surgical management of pain
- destroy dorsal root of spinal nerves |
|
|
Miosis
|
constricted pupil
|
|
|
mydriasis
|
dilated pupil
|
|
|
What two structures in the eye are responsible for accommodation?
|
Ciliary muscle and Zonule Fibers (suspensory ligaments)
|
|
|
Optic disc
|
- only axons of retinal ganglion cells
- no photoreceptors = blind spot - retina overlaying lamina cribrosa |
|
|
Fovea
|
highest visual acuity
- no rods, only cones - surrounded by macula - 1.5 mm diameter - all layers (except for photoreceptors) are pushed laterally so they don't interfere with photoreceptors - on ophthalmoscope = area without blood vessels |
|
|
Macula
|
- region of high acuity
|
|
|
Refractive Power
|
1/ focal length
unit = Diopter (D) |
|
|
Far vision
|
Ciliary Muscle - Relax
Zonule fiber - Tighten Lens = Flat - ↓ Refractive Power (13D) F - Far, Flat |
|
|
Near Vision
|
Ciliary Muscle Constricted
Zonule Fiber - Loose Lens = - Rounded (more convex) - ↑ refractive power (26D) |
|
|
Presbyopia
|
- inability to focus on near objects with age
- lens loose its elasticity with age |
|
|
What is normal visual acuity?
|
an angle of 5 minutes of a degree
|
|
|
What factors determine visual acuity?
|
- low threshold for 2 point discrimination
- high density of photoreceptors - proper functioning accommodation apparatus |
|
|
Emmetropia
|
normal sightedness
- |
|
|
Papilledema
|
Optic Disk Edema
- Indicates ↑ Intracranial Pressure - compromised venous drainage = dilated retinal veins = optic disk pushed forward and appears white (instead of pink) |
|
|
scotoma
|
areas of lost visual acuity due to retinal detachment
ie. dead pixels |
|
|
When looking at the ocular fundus, the optic disc is always on the ______ side.
a. Lateral b. Medial |
b. medial side.
|
|
|
Macular Degeneration
|
- loss of vision in macula due to damage to retina
- Neo-vascualr = abnormal blood vessel growth. Most severe type. - Treatment: Laser Photocoagulation - Leading cause of vision loss in US |
|
|
Temporal Resolution
|
ability to distinguish subsequent stimuli from each other (time related)
|
|
|
Spatial Resolution
|
ability to distinguish adjacent stimuli from each other (relating to space)
|
|
|
Neurotransmitter for Photoreceptors
|
Glutamate
|
|
|
Which second messenger keeps the Na+ channel open in the dark current?
a. cAMP b. cGMP c. IP3 |
cGMP
|
|
|
True/False
More glutamate is released from photoreceptors in the dark. |
true.
|
|
|
There is more convergence in:
a. Rods b. Cones |
Rods have more convergence.
Cones have less convergence = better visual acuity |
|
|
Retinal
|
- light absorbing substance
- derived from Vitamin A |
|
|
Superior Visual Field
|
- temporal radiation
- below the calcarine sulcus |
|
|
Infereior Visual Field
|
- parietal radiation
- above the calcarine sulcus |
|
|
Sparing of the macula
|
- vascular damage usually involves posterior cerebral artery
- the macula region is also supplied by the middle cerebral artery and therefore it is usually spared |
|
|
Color Agnosia
|
inability to distinguish color hue
- lesion in area 17 or 18 |
|
|
Ocular Dominance columns
|
organization of primary visual cortex
- arranged in alternating contralateral/ipsilateral columns |
|
|
Orientation columns
|
- primary visual cortex shows preference for certain stimuli
- ie. horizontal or vertical |
|
|
Magnocellular Pathway
|
- Dorsal (parietal) pathway
- Depth & Motion (answers Where?) (Location & Movement) - detects fast moving stimulus - synapse in first 2 layers of lateral geniculate nucleus - Magno b/c larger cells |
|
|
Parvocellular Pathway
|
- Ventral (Temporal) pathway
- Form & Color (answers What?) - Color vision and acuity - projects deeper into the primary visual cortex - synapses in layers 3-6 (deeper and in more layers than magnocellular pathway) |
|
|
otosklerosis
|
replacement of normal bone in labyrinth and stapes footplate with lamellar new bone
= fusion of stapes with border of oval window. = conductive hearing loss ~ 40db |
|
|
Vestibular schwannoma
|
Causes sensorineural hearing loss
- benign tumor from Schwann cells - compresses vestibulo-cochlear nerve within internal meatus - symptoms = sensorineural hearing loss and tinnitus |

