![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
189 Cards in this Set
- Front
- Back

|
RUP in For; recurrence, unknow primary, progression on chemo, instable spine, fx.
InSSS (insensitive to radiation, no paraplegia >48hrs, single level, systemic tumor limited). |
|
|
Intradural Extramedullary Tumors
Extramedullary tumors can cause radicular pain or vertebral pain (focal pain localized to area of spinal cord compression by the tumor); extramedullary lesions may also cause Brown-Séquard syndrome. |
t
|
|
|
1)Nerve sheath tumors: most common intradural extramedullary tumors.
|
Neurofibroma, schwannoma, ganglioneuroma, neurofibrosarcoma.
|
|
|
Neuroimaging: hyperintense on T2-weighted images, strong enhancement apparent on T1-weighted images with contrast (periphery of tumor may be more intensely enhancing than the center).
|
B.C of the chronicity and slow growth of the tumors, osseous changes such as erosion and enlargement of neural foramina may be seen.
d.Schwannomas may have a cystic component and, less frequently, a hemorrhagic or necrotic component |
|
|
2)Meningiomas
a.Predilection for thoracic spine. Because that is where the majority of the dura located. |
3)Paragangliomas
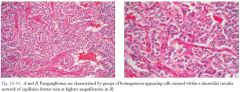
a.Arise from filum terminale or one of lumbosacral roots of cauda equine. b.Well-encapsulated, highly vascular tumors appearing hyperintense on T2-weighted images and strongly enhance on T1-weighted images with contrast, with flow voids (which enhance with contrast) above and below tumor (Fig. 16-33) c.Pathology: well-encapsulated vascular neoplasm; sheets of uniform cells with granular cytoplasm surrounded by delicate fibrovascular network |
|
|
4)Dermoid tumors:
a.Congenital midline cystic lesion often occurring in lumbosacral spine and conus medullaris. b.May be intramedullary or intradural extramedullary tumor. c.Same appearance as fat on neuroimaging. |
5)Epidermoid:
a.Congenital or acquired (trauma or late complication of lumbar puncture). b.Symptomatic cases present with radiculopathies and gait abnormalities |
|
|
t
|
|
|
Intradural Intramedullary Tumors?
|
1-Ependymoma
2-Myxopapillary ependymoma 3-Astrocytoma 4-Intramedullary spinal cord metastasis (ISCM) |
|
|
What is the Most common intramedullary spine tumor in adults?
|
Ependymoma
|
|
|
2)Myxopapillary ependymoma: most common tumor of filum terminale and cauda equine.
3)Well-demarcated slow-growing tumors, may erode bone. 4)May have cystic or hemorrhagic components |
t
|
|
|
Where does Myxopapillary ependymomas arise from ?
|
ependymal cells in fibrous band of filum terminale.
|
|
|
s/s of ependymoma?
|
a.Back pain (most common), neck pain, sphincter dysfunction, dysesthesias, leg weakness.
b.Funicular (central) pain characteristic of intramedullary expansile masses: deep and poorly localized dysesthetic discomfort that may be related to involvement of spinothalamic tracts or dorsal columns |
|
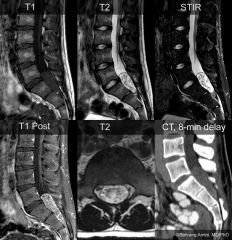
a.Isointense with spinal cord on T1-weighted images and hyperintense on T2-weighted images; enhancement is heterogeneous and intense.
b.Commonly a rim of hypointense signal surrounding the intramedullary tumor on T2-weighted images. |
Tend not to invade adjacent normal tissue and may be easily resected.
12)Postresection radiotherapy may be given for residual tumors but usually is not recommended. |
|
|
Intra-medullary astrocytoma?
|
1)Most common intramedullary spine tumors in children (twice as common as ependymomas in children).
2)Intratumoral cystic component or syringomyelia in up to 40% of tumors. 3)Most are low grade and slow growing (in contrast to intracranial astrocytomas). 4)Cervical cord (then thoracic) is most common site. 5)Most common presentation: focal axial pain, followed by weakness and paresthesias |
|
|
Intramedullary spinal cord metastasis (ISCM)
|
Most often affects conus medullaris.
Small cell lung carcinoma (MC), breast cancer, lymphoma, colorectal carcinoma, renal cell carcinoma and melanoma. Usually in the setting of extensive systemic disease. 4.Equally distributed between cervical, thoracic and lumbar areas. 5.Reach the cord through either hematogenous spread or via leptomeninges or along nerve root or Virchow-Robin spaces. |
|
|
the weakness develop concomitantly or shortly after pain development (very unusual to have weeks or months interval).
7.Start at the dorsolateral cord at the border zone of circulation between anterior and posterior spinal arteries. 8.Commonly have sacral sparing sensory loss. 9.Early sphincter dysfunction. 10.Also present as brown-Sequard. 11.Dx based inferred in patient with metastatic cancer and enhancing lesion in the spinal cord. |
Prognosis: usually poor.
Rx: 1)Fractionated focal radiation is the treatment of choice as most cases are palliative (aim just for neurological improvement). 2)Surgical resection in: a.Limited systemic malignancy b.Radioresistent tumor. |
|
|
Radiation myelopathy?
|
Early myelopathy (2-6 months) are usually self limited and mild.
Should be checked for compression for tumor related swelling or pathological Fx. These conditions response to steroid or surgical decompression if necessary. |
|
|
Pathogenesis: radiation induced demyelination of posterior column.
Lhermitte phenoma is common after radiation. MR is normal. |
No increased risk of late radiation induced myelopathy
|
|
|
Late radiation myelopathy: several months to years, usually progressive.
>1 yr. In 1% to 5%. May start as brown-Sequar and progress to paraparesis or quadriparesis. MRI: Mb normal or shows hi T2 signal with patchy enhancement. Corticosteroid is often tries, ? role for Bevacizumab. |
Risk of myelopathy depend on both total dose of radiation and the fraction size.
Vulnerable cells include: vascular endothelial cells and oligodendrocyte. |
|
|
Chemotherapy induced myelopathy?
|
Most likely to be seen in drug administered into CSF such as MTX, cytarabine and theiotepa.
Lhermitte phenomena happen after IV cisplatin. |
|
|
PERIPHERAL NERVE TUMORS ?
|
1)Schwannoma 2)Neurofibroma
3)Perineuroma 4)Malignant Peripheral Nerve Sheath Tumor 5)Triton Tumo |
|
|
Schwannomas?
|
1)Most common nerve sheath tumors.
2)Benign (often single) tumors. 3)Peak incidence in fourth to fifth decades of life. 4)Higher incidence in women. 5)Usually present as asymptomatic, often superficial and palpable mass with Tinel’s sign (pain less common). |
|
|
Where does schwannoma arise from?
|
1)Cranial nerve
a.Most common: CN VIII, “acoustic neuroma,” an enhancing lesion that may or may not have cystic component. b.Bilateral acoustic neuromas are suggestive of NF2. |
|
|
2)Spinal nerve root
a.Extramedullary intradural (sometimes extradural), appear as dumbbell-shaped tumor as it encases spinal cord and expands through foramina bilaterally. b.Spinal schwannomas may occur at multiple levels in patients with NF2. c.Signs and symptoms of radiculopathy, myelopathy, etc. |
3)More distal peripheral nerve location.
|
|
|
4)Plexiform schwannomas are usually cutaneous in location and rare
|
t
|
|
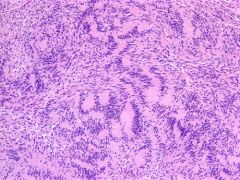
Schwannoma. Palisading tissue pattern
|
1)Antoni A pattern (spindle-shaped cells arranged in compact fascicles with dense reticulin) and Antoni B pattern (stellate- or spindle-shaped cells arranged in loose, myxomatous, microcystic stroma with sparse reticulin), and Verocay bodies (appearing as columns of nuclei present in the palisading arrangement of Antoni A pattern).
2)May be hyalinized, thickened blood vessels 3)Carney’s complex: melanotic schwanoma(psammoma bodies). 4)Stain: +ve S-100 and focal GFAP |
|
|
Mx of schwannoma?
|
1)Asymptomatic tumors may be followed with serial imaging; resection is considered if there is growth or increasing neurologic symptoms; recurrence is rare.
2)Malignant transformation of the tumor is rare (much less common than that of neurofibroma). |
|
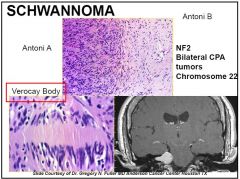
Antoni A pattern (spindle-shaped cells (tapered end) arranged in compact fascicles with dense reticulin) and Antoni B pattern (stellate- or spindle-shaped cells arranged in loose, myxomatous, microcystic stroma with sparse reticulin), and Verocay bodies (appearing as columns of nuclei present in the palisading arrangement of Antoni A pattern).
|
2)May be hyalinized, thickened blood vessels
Carney’s complex: melanotic schwanoma(psammoma bodies). Stain: +ve S-100 and focal GFAP |
|
|
Neurofibroma?
|
1)Higher incidence in women
2)Often present with pain, dysesthesias, or paresthesias in the distribution involved nerve, often with Tinel’s sign. 3)May be cutaneous or plexiform (intraneural). 4)Associated with NF1 |
|
|
Where does neurofibroma arise from?
|
Likely arise from perineural fibroblasts
|
|
|
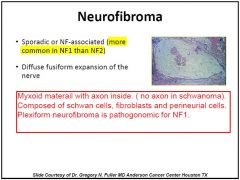
Microscopic features (Fig. 16-36 B)
1)Haphazard arrangement of spindle-shaped cells in myxomatous- mucoid stroma or in between collagen fibers, infiltrating nerve fascicles (not displacing them as do schwannomas). |
2)Blood vessels tend to be less hyalinized and thickened than schwannomas.
•Asymptomatic lesions that remain stable need to be followed because they can undergo malignant transformation (5%-10% of tumors). •Low threshold for surgical resection is often practiced for symptomatic tumors (pain, neurologic deficits) or for growing tumor |
|
|
t
|
|
|
What is Neurothekomas?
|
Benign, slow-growing soft, mobile nerve sheath tumors often located in the dermis, may originate from Schwann cells or perineural cells.
2)Peak incidence: first three decades of life 3)Recurrence is rare |
|
|
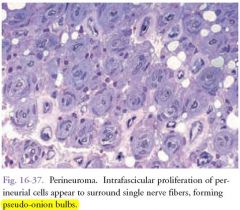
Intraneural Perineuromas:
1)Rare, benign masses arising in extremities. 2)Peak incidence: first two decades of life. 3)Patients may present with motor mononeuropathy. 4)Affected nerve becomes enlarged |
Microscopic features: intrafascicular proliferation of perineurial cells appearing to surround single nerve fibers, forming pseudo-onion bulbs .
Curative treatment: complete resection |
|
|
Malignant Nerve Sheath Tumors?
|
May arise de novo or from malignant transformation (usually of plexiform neurofibroma, much less commonly schwannoma).
2)Associated with NF1. 3)Usual presentation: pain, often with progressive neurologic deterioration and rapidly growing mass. |
|
|
Rx of MNST?
|
1)Wide local resection (limb amputation may be needed)
2)External radiotherapy (with or without chemotherapy |
|
|
Prognosis of MNST?
|
1)High recurrence rate despite therapy
2)5-year survival rate: 10% to 50% 3)Distal metastasis is rare 4)Patients with NF1 usually have worse prognosis |
|
|
|
|
|
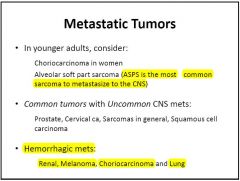
Features of mets?
|
|
|
|

|
|
|
Lung cancer account for the majority of metastasis.
Melanoma has the highest propensity to disseminate to the brain (50% of advanced melanoma develop brain metastasis). |
10% of metastasis present with hemorrhage (particularly melanoma, renal cell carcinoma, thyroid carcinoma and choriocarcinoma).
|
|
|
Mets distribution?
|
Distribution reflect blood flow and volume:
80% occur in cerebral hemisphere. 15% cerebellum. 5% in brainstem. |
|
|
Mets pathophysiology?
|
Cells escape from tumors. Invade surrounding tissue into blood stream. Extravasation and proliferation in secondary organs. Cells tends to arrest at grey white junction and watershed zones where Brain vasculature narrows substantially. Arrest depends on : 1) Tumor cell recognition of specific endovascular surface
2) size. Cells has to leave the capillary beds and enter brain parenchyma = extravasation. Growth of the tumor cells depend on adequet blood supply. (angiogenesis formation of blood vessels from sprouting) |
|
|
Most common tumors to metastasize to skull (in descending order of frequency)?
|
B LP RT breast> lung> prostate> renal> thyroid> melanoma.
|
|
|
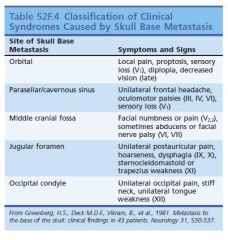
Skull mets:
|
2)Half of cases are asymptomatic (especially calvarialbased metastases).
3)Calvarial metastases may produce focal neurologic symptoms related to focal area of involvement. 4)Invasion of cerebral venous sinuses predisposes to venoocclusive disease and related complications such as venous infarction and increased ICP. 5)Tumor extent and involvement of underlying parenchyma are better delineated with GAD enhanced MRI. 6)RT often used for symptomatic Pt. |
|
|
Most common cancers to cause dural metastasis?
|
non–small cell carcinoma, prostate cancer, breast cancer.
|
|
|
How would dural mets become symptomatic?
|
become symptomatic by compressing adjacent parenchyma or venous sinuses or by producing subdural hygromas or fluid collections
|
|
|
Rx options for dural mets?
|
1)Often focal radiotherapy
2)Whole-brain radiation reserved for leptomeningeal metastasis. 3)Surgical resection is often indicated for large symptomatic tumors. |
|
|
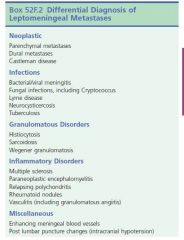
Tumors associated with Leptomeningeal Metastases?
|
Solid tumors esp. adenocarcinomas such as breast, lung, and gastrointestinal), non-Hodgkin’s lymphoma, and leukemia.
|
|
|
Once leptomeninges are involved, metastatic cells can spread via CSF; have a propensity for cauda equina and basal cisterns because of gravity and slow CSF flow
|
t
|
|
|
4)Tumor cells may invade Virchow-Robin spaces; with extensive perivascular infiltration, tumor nodules may take on a “miliary” pattern
|
When subarachnoid space is involved, presentation is often that of carcinomatous meningitis, symptoms related to cranial nerve (most commonly CNs III, IV, and VI) or spinal root (most commonly lumbosacral) involvement, and hydrocephalus, which may result from occlusion of CSF outflow by tumor cells
|
|
|
6)Positive CSF cytologic findings are diagnostic and helpful; may also occur with parenchymal metastases without leptomeningeal involvement
|
a.CSF examination yield can be increased from 50% with first lumbar puncture to about 90% with repeated punctures.
b.In other 10% of cases, malignant cells are often strongly adherent to leptomeninges and do not shed into CSF. |
|
|
Tumors associated with multiple metastases?
|
Lung cancer and melanoma are most likely to produce multiple metastases
|
|
|
5)Renal cell carcinoma, breast cancer, and colon cancer are most likely to produce single brain metastasis.
6)Retroperitoneal, pelvic, gastrointestinal primary tumors have predilection for posterior fossa |
Up to 75% of patients have neuroimaging evidence of multiple metastases at presentation
|
|
|
For patients presenting with brain metastasis with no known primary tumor?
|
a.Most common type of primary neoplasm: non–small cell carcinoma.
b.Next most common: breast cancer, small cell carcinoma, malignant melanoma (second, third, and fourth most common, respectively). c.These are followed by renal cell carcinoma and gastrointestinal tract cancers |
|
|
Most common tumor with metastasis to brain in children?
|
neuroblastoma, Wilms’ tumor, sarcomas
|
|
|
Neoplasm with highest tendency for CNS metastasis?
|
malignant melanoma
|
|
|
Why Metastasis occurs with hematogenous spread and typically land at gray matter–white matter junction ?
|
because of abrupt change in blood vessel caliber.
|
|
|
11)Except for certain neoplasms such as malignant melanomas that can penetrate arterial (arteriolar) walls and are often hemorrhagic, most tumors usually penetrate capillary beds to arrive at their destination.
|
Malignant melanoma, choriocarcinoma, non–small cell lung carcinoma, and renal cell carcinoma are most common metastatic malignancies that have a tendency to present as hemorrhagic lesions.
|
|
|
Brain mets s/s?
|

1)Incidental finding in about 1/3 of patients.
2)5-10% present simultaneously with primary and secondary brain Mets (synchronus presentation). 3)10% present as ICH. 4)Seziures happen in 20-25% of brain Metz |
|
|
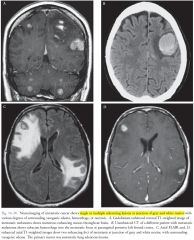
Neuroimaging of brain mets?
|
1)Single or multiple lesions at the gray matter–white matter junction with various degrees of surrounding vasogenic edema, hemorrhage, or necrosis.
2)Most often appear hyperintense on T2-W images and hypointense on T1-weighted images (except for hemorrhage). 3)Most metastases strongly enhance (often ring enhancement, sometimes enhancement of entire lesion, which is frequently heterogeneous). |
|

|
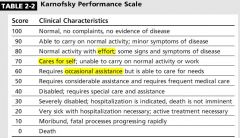
Prognosis:
Median survival = 6 months |
|

|
|
|
|
4 FACTORS to assess survival in pt with mets?
|

|
|
|
Special Favorable prognostic factors:
1)Breast Ca with +ve ERBB2. 2)NSCLC with EGFR |
t
|
|
|
Management of Metastatic Neoplasm
1)search for primary tumor. 2)< 1cm can be closely monitored without affecting the success of subsequent therapy. 3)Factors affect ttt: size , location , functional status, primary tumor control. 4)Goal of treatment: improve quality of life and improve or stabilize neurologic condition |
Corticosteroid therapy (lymphoma must be reasonably excluded).
6)Reduces peritumoral vasogenic edema and induces rapid improvement in symptoms. 7)Corticosteroids improve median survival by 1 or 2 months and should always be used. 8)Many authorities prefer dexamethasone to other corticosteroids. |
|
|
9)Except for metastatic melanoma, tumors involving motor cortex, and parenchymal tumors with leptomeningeal involvement, anticonvulsant therapy is often not indicated as prophylactic treatment
|
10)Treatment often involves resection of surgically accessible single lesion, followed by radiation.
|
|
|
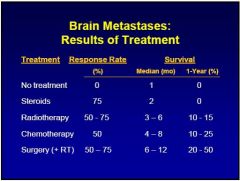
Radiotherapy
1)Marginal improvement in overall survival rates 2)Important role in tumor shrinkage and palliation |
3)Whole-brain radiation often given to pt with radiosensitive tumors with multiple metastases such as non–small cell carcinoma of lung.
4)Total dose of 30 Gy to 40 Gy(daily fraction = 2 Gy) Gy= Gray. 5)WBRT: increase survival from 1-2 Mo to 3-6 Mo |
|
|
Radiosensitive tumor GBS (continuum)?
|
1)SCLC.
2)Breast Ca. 3)Germ cell tumor |
|
|
7)Radioresistant: MRS?
|
1)Melanoma.
2)Sarcoma. 3)Renal cell Ca. |
|
|
Radiosensitizers:
|
1)NSCLC: motexafin gadolinium.
2)Breast Ca: efaproxiral. 3)Melanoma:Temozolomide. 4)Others: lonidamine, misonidazole, bromodeoxyuridine |
|
|
One way to reduce neurotoxicity?
|
<3 Gy daily significantly reduce the risk of neurotoxicity.
|
|
|
Role of Prophylactic cranial RT?
|
reduces risk of metastasis and increases survival rates; should be considered for radiosensitive tumors that commonly metastasize, such as small cell lung carcinoma.
|
|
|
Stereotactic radiosurgery
1)3 delivery systems: a.Linear accelerator (hi energy X-ray). b.Gamma knife 9gamma radiation). c.Cyclotron (protons). |
2)Indications:
a.Reserved for lesions with max diameter of 3 cm. b.Surgically un accesable lesions (midbrain, basal ganglia). 3)Local control is 70-90% at 1 yr. |
|
|
4)Complications of SRS: SER
a.Edema. b.Sz. c.Radiationnecrosis. 5)Prolongs survival similar to surgical resection and Radiotherapy |
Not ideal for large or radioresistant tumors
|
|
|
Surgery for single lesions: SRS Vs SRS + WBRT Vs WBRT: all equivalent except in single mets WBRT is inferior (7Vs 5 Months), combination therapy has better local control and KPS.
WBRT Vs WBRT + Surg (single Mets): Patchell: WBRT 15 weeks (3M) Vs WBRT+ surgery 40 weeks (11 mo). |
Surgery Vs WBRT + Surg. (single Mets)
Patchell for single Mets; better local abd distant dss control but no difference in survival SRS Vs Surgery: 3 studies shows no difference while 1 studies shows better survival with surgery. If tumor is big: surgery MB better as it provide faster edema control. |
|
|
Role of chemo for mets?
|
1)Questionable role particularly with BBB.
2)Sometimes not effective because pt may have already received chemotherapy for treatment of primary tumor. 3)Temozolomide, with high CNS penetration, is sometimes used for brain metastases. |
|
|
Small cell lung carcinoma: surgical resection, radiotherapy, prophylactic cranial radiation, and possibly chemotherapy.
|
Non–small cell lung carcinoma and breast cancer
a. Surgical resection for single, surgically accessible metastasis, followed by radiation (continuing controversy about whole-brain radiation vs. stereotactic radiation vs. both). b. Radiation without surgical resection for multiple metastases. c. Platinum Mb be helpful. d. EGFR inhibitors (erlotinib and gefitinib). e. angiogenesis inhibitors (bevacizumab) anti-VEGF f. Tyrosin Kinase receptor inhibitors (sorafenib) and sunitinib |
|
|
Breast ca:
Risk factors for mets (Hall Le)? |
1)Hormone receptor negative tumors.
2)Young age. 3)Hi LDH. 4)L.N positive. 5)Lymphovascular invasion. 6)ERBB2 overexpression |
|
|
In breast ca: Response rate of 43% to 59% in pts treated with cyclophosph/5FU/prednisone or 5FU/prednisone/MTX/vincristine and 5FU/MTX.
Lapatinib (tyrosine kinase inhibitor and ERBB2 inhibitors. |
t
|
|
|
Melanoma mets?
|
1)Surgical resection for single, accessible tumors.
2)This may be followed by postoperative radiotherapy, which may be palliative and slow neurologic progression, despite radioresistance of the tumors. 3)Melanoma tends to be chemotherapy-resistant. 4)Possible role of fotemustine (nitrosourea; with good BBB penetration) 5)Ipilimumab: anti-CTLA-4 monoclonal antibx approved for metastatic melanoma |
|
|
Mx of multiple mets?
|
1)Whole-brain radiation is typically recommended; however, can consider stereotactic radiation of individual lesions, depending on number of lesions or targets.
2)Surgical resection (as palliative treatment) of symptomatic lesions may improve quality of life and survival. |
|
|
|
|
|
Leptomeningeal Metastasis:
Pathogenesis? |
Direct extension from parenchyma or dural metastasis.
2)Hematogenous spread. 3)Venous system (leptomeningeal veins). 4)Perineural extension. 5)Virchow-Robin spaces. |
|
|
MCC sites: basal cisterns, dorsal aspect of spinal cord particularly cauda equine
|
Common cancers: leukemia, lymphoma, breast ca, lung ca and melanoma
|
|
|
Leptomeningeal mets median survival?
|
Female predominance because of breast cancer.
Median survival is 2 yrs for solid tumors and 11 months for hematological malignancy |
|
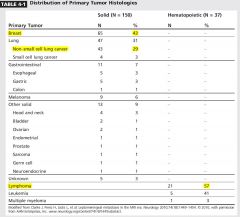
|
Breast 40% . NSCLC 30
|
|
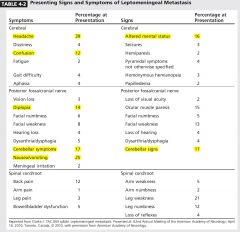
|
Headache 40% > Neusea and vomiting 25%.
Sings ( cerebellar >confusion |
|
|
Dx of leptomeningeal mets?
|
MR for entire neuro-axis.
Enhancement can be linear but often has irregularity or nodularity. More sensitive for solid tumor 76%-100% CSF cytology sensitivity: 71%.(90% after 3rd sample). |
|

CSF changes in LM mets?
|
1)Hi protein.
2)Low glocuse. 3)N CSF pleocytosis (T or B lymphocytes). 4)Measure CEA in CSF. 5)>1% of the serum level of tumor marked in CSF is diagnostic for leptomeningeal metastasis. |
|
|
Treatment of leptomeningeal metastasis?
|
Usually palliative.
If the goal is eradication of leptomeningeal tumor then the whole neuroaxis should be eradicated. Complications of eradicating the whole neuroaxis: GBM 1)Gastrointestinal toxicity. 2)B.M suppression. 3)Mucositis |
|
|
Systemic chemo for LM mets?
|
Limited by penetration of dg to CNS.
Hi systemic MTX (3-8g/m3 or cytarabine hi dose allow for therapeutic level in CSF. Temozolomide and capecitebine and thiotepa penetrate into CNS. |
|
|
Intrathecal chemo for LM mets?
|
Options: MTX
cytarabine thiotepa sustained release cytarabine |
|
|
Side effect to Intra-thecal chemo?
|
aseptic meningitis. Acute encephalopathy (transient), transverse myelopathy, chronic leukoencephalopathy.
|
|
|
Contraindication of intrethecal chemo?
|
If pt need VP shunt because of the potential of intraperitoneal toxicity
|
|
|
LM mets prognosis?
|
2-3 months. (solid tumor 2.3 M for hematological Ca 4.7 months
|
|
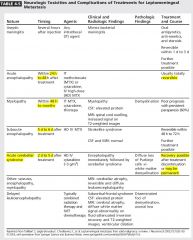
|

|
|

|

|
|

|
t
|
|
|
MOA of Cisplatin?
|
•Platinum-based alkylating agent, renally excreted, may cause renal toxicity.
|
|
|
Neurotoxicity of cisplatin?
|
•Neurotoxicity: GVL (ganglio, vestibule, Lhermitte, Leukoencephalo)
1)Peripheral neuropathy and ganglionopathy (axonal and demyelinating). 2)Ototoxicity and vestibulopathy. 3)Lhermitte’s sign in absence of other myelopathic features. 4)Rarely, reversible leukoencephalopathy |
|
|
MOA of MTX?
|
Antifolate agent that acts to inhibit dihydrofolate reductase; may cause kidney or liver toxicity.
used in treatment of PCNSL, primary sarcomas of CNS, leptomeningeal metastasis. |
|
|
MTX neurotoxicity? MEEMSC
|
1.aseptic meningitis (with intrathecal administration),; nuchal rigidity, lymphocytic pleocytosis and Hi protein.
2.acute encephalopathy (may be marked by transient symptoms, normal MRI, slow electroencephalogram [EEG]), 3.chronic leukoencephalopathy (most common delayed complication with variable degrees of dementia, diffuse white matter disease and atrophy), severe form cause disseminated necrotizing encephalopathy (nonenhancing diffuse periventricular White matter lesions). 4.myelopathy |
|
|
MOA of Cytarabine (cytosine arabinoside, ARA-C)?
|
1)Pyrimidine analogue; active metabolite inhibits DNA polymerase A and chain elongation.
2)Used to treat lymphoma, leptomeningeal carcinomatosis, leptomeningeal lymphoma; intrathecal administration ideal for leptomeningeal neoplastic disease |
|
|
Cytarabine neurotoxicity?
|
1.Acute cerebellar syndrome with variable degrees of truncal and gait ataxia occurs in 10% to 25% of cases.
2.Aseptic (chemical) meningitis and reversible myelopathy (rapidly ascending flaccid paralysis, sensory level and sphincter dysfunction) with intrathecal route are rare. 3.transient encephalopathy |
|
|
Vinca Alkaloids (e.g., vincristine) MOA?
|
inhibition of microtubule assembly and arrest of cell cycle.
2)Effective for lymphomas, leukemia, sarcomas; vincristine used in oligodendrogliomas (vincristine is part of PCV regimen for oligodendroglial tumors). |
|
|
Vinca Alkaloids (e.g., vincristine) NEUROTOXICITY?
|
a.Primarily axonal sensorimotor peripheral neuropathy, often painful neuropathies.
b.Vincristine may also produce autonomic neuropathy, cranial neuropathies, and, less commonly, (SIADH) and related hyponatremia. c.Vinblastine, vindesine, and vinorelbine are less toxic. |
|
|
Fluorouracil (5 FU) MOA?
|
1. Inhibits thymidylate synthase and disrupts DNA Synthesis.
2. Used to treat breast cancer and colon cancer |
|
|
Fluorouracil (5 FU) NEUROTOXICITY?
|
5% of Pt may have acute cerebellar syndrome characterized by acute onset of appendicular & axial/midline (gait) ataxia (including cerebellar dysarthria, which may occur several weeks to months after initiation of treatment).
2)These symptoms usually are completely reversible with discontinuation of the agent. |
|
|
MOA of Procarbazine?
|
•Part of PCV regimen for oligodendroglial tumors.
•Inhibitor of monoamine oxidase; may not be used concomitantly with tyramine-rich diet or tricyclic antidepressants |
|
|
Procarbazin neurotoxicity?
|
•Oral dose may produce mild reversible encephalopathy and intravenous route may yield severe encephalopathy.
•Other side effects include peripheral neuropathy and autonomic neuropathy. •These adverse effects are usually transient and/or reversible. |
|
|
Nitrosoureas?
|
•Lipid-soluble alkylating agents that easily cross BBB.
•Carmustine (BCNU), lomustine (CCNU). •May be used in treatment of high-grade gliomas or glioblastoma and lymphoma. |
|
|
Nitrosoureas neurotoxicity?
|
•High-dose IV carmustine may cause encephalomyelopathy and seizures.
•Intra-arterial carmustine may cause retinopathy and blindness, headaches, and progressive encephalopathy |
|
|
Ifosfamide (alkylating agents) ?
|
causes acute encephalopathy (confusion, hallucinatin Sz and drowsiness).
a.Usually pts recover in 48-72 hrs. b.IV methylene blue may shorten the duration. |
|
|
2.Busulfan: causes generalized Sz. (dilanitn increases its clearance).
3.MTX Hi dose IV --encephalopathy within 24hrs and resolve spontanously . |
4.cytarabine causes acute cerebellar syndrome: truncal and gait ataxia. Should be discontinoued immediately.
|
|
|
PRES: MC affect parietal and occipital followd by frontal lobe.
Reduced sympathetic innervation in posterior circulation which causes dysregulation. PRES: MC affect parietal and occipital followd by frontal lobe. Reduced sympathetic innervation in posterior circulation which causes dysregulation. |
t
|
|
|
Drugs ass e PRES: CCC GGBS like
|
1.Cisplatin
2.Cyclophosphamide 3.High-dose corticosteroids 4.L-asparaginase 5.Gemcitabine 6.Bevacizumab (VEGF inhibitor). 7.Sorafenib (VEGF inhibitor). 8.Growth factors |
|
|
Cognitive dysfunction: (Chemobrain and chemofog)
Risk factors |
1.age <5 yrs.
2.female gender. 3.level of cognitive function prior to chemo. 4.Concurrent toxic or metablic abn. 5.Fatigue. |
|
|
Cognitive dysfunction can be limited or progressive.
Imaging can be normal. Pattern Is consistent with preferential involvement of frontal and subcortical white matters. |
??Erythropoietin, methylphenidate, modafinil, cholinesterase inhibitors, nonsteroidal anti-inflammatory agents
|
|
|
Cerebrovascular events More common with: (CBSS LiM)
|
1.Cisplatin.
2.Bevacizumab (VEGF inhibitor). 3.Sunitinib. (VEGF inhibitor) 4.Sorafenib. (VEGF inhibitor) 5.L-asparaginase. 6.Imatinib. 7.Methotrexate |
|
|
L-asparaginase indications and MOA
|
Used to ttt ALL.
Deprive leukemic cells from asparagine an essential amino acid |
|
|
L-asparaginase Complications
|
Ass e coagulation defects ---bleed and thrombotic events.
Ass e venous sinus thrombosis. TTT: anticoagulation (risk of bleed is low even with Hi grade glioma or metastasis). Also safe to combine bevacizumab with anticoagulation. (VEGF inhibitor) ass e hemorrhage and thromboembolic stroke. 3 folds increase risk of stroke |
|
|
Peripheral neuropathy classes associated with Peripheral neuropathy
|
1.Platinu agents (eg, cisplatin, oxaliplatin),
2.Taxanes (eg, paclitaxel) 3.Vinca alkaloids (eg, vincristine, vindesine) 4.Bortezomib. 5.Thalidomide |
|
|
Acute infusion-related neuropathy described as tingling or burning in the extremities, throat, mouth, or face that can be induced by contact with cold surfaces or liquids.
|
Improve with reducing the dose or stopping the medications
|
|
|
Coasting effect; cisplatinum induced PN continue to worse few months later.
Caosting effects occur with Cisplatinum >>> paclitaxel and vincristine. |
Hodgkin and SCLC ass e paraneoplastic sensopry neuronopathy: usually asymmetric and rapidly progressive paresthesia and pain.
Look for plasma cell disords (paravertebral plasmacytoma). Vincristine should be avoided in all pt with Charcot Marie tooth. Calcium and Magnesium infusion may decrease the incidence of oxiplatin induced chronic sensory neuropathy. TCA, Gabapentin, Lamotrigine failed to show benefit in RCT. |
|
|
Chemo associated with exacerbate myasthenia gravis or lead to the development of a myasthenic syndrome
|
Doxorubicin, cisplatin, etoposide, dexamethasone, interferon alfa, interleukin-2, and etanercept
|
|
|
Neurological complications and use of Imatinib:
|
Oral tyrosin kinase, Inhibitor of bcr-abl.
Causes muscle cramp and myalgia. |
|
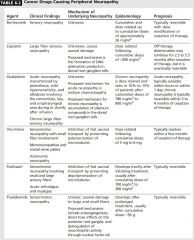
|
t
|
|
|
COMPLICATIONS OF RADIOTHERAPY
|
Usually Dose-Dependent
Total dose, daily fractions, and Rx duration are proportional to radiation-induced injury in sigmoid shaped curve (small increments above critical level greatly increase rate of cerebral necrosis). Tissue and vascular injury is unavoidable so no safe RT dose has been identified. |
|
|
Indication for focal EBRT (external beam RT)
|
1.Primary parenchymal tumor
2.Pituitary tumor 3.Meningeoma |
|
|
Indication for WBRT:
|
1.Treat or prevent brain or leptomeningeal metastasis.
2.medulloblastoma 3.germinoma 4.primary CNS lymphoma. |
|
|
RT complications: Encephalopathy and others
1)Acute |
1.Sudden onset of altered mental status, HA, fever, and possibly worsening of neurologic deficits attributable to tumor.
2.Presumably result of diffuse edema, and tends to occur in pt with large tumors receiving high doses of whole brain radiotherapy. |
|
|
Rx of post RT acute encephalopathy, what is the prognosis
|
3.Acute encephalopathy should be treated with high doses of dexamethasone.
4.Prophylaxis includes administration of dexamethasone to patients with large tumors before large doses of wholebrain radiation. 5.Pt should be reassured that it is transient |
|
|
Associated features with post RT acute encephalopathy
|
Can be accompanied with:
1)Fever 2)Nausea 3)Vomiting 4)Focal deficit. |
|
|
Post-RT Early delayed (subacute):
|
Characterized by worsening of neurologic function, HA, fever, somnolence, several months after completion of RT.
2.This is characterized by worsening edema and mass effect, sometimes with increased enhancement (particularly in focal RT with Temozolamide). this phenomena is called pseudoprogression. |
|
|
this phenomena is called pseudoprogression.
Similar imaging abn with neuro signs occur in 50% of pts treated with SRS. Pseudo progression is a clinical diagnosis. Rule out other causes of neurological worsening: AED, metabolic derangements, mood disorders and infection |
3.This complication usually occurs about 3 to 8 weeks after completion of radiotherapy (within 6 months).
|
|
|
What is Somnolence syndrome
|
fatigue, impaired cognition and new or worsening deficit. Mech: exaggerated response to RT or transient demyelination.
Ttt: steroid or rarely lesion resection is required. Close monitoring to make sure it has resolved. |
|
|
Late delayed complications of RT
|
1.Leukoencephalopathy: Chronic progressive dementia with deficits primarily of attention and problem-solving, also gait disturbance, urine incontinence, occasionally diffuse atrophy, ventriculomegaly, white matter changes.
2.Risk is higher with daily radiation >2Gy. |
|
|
3.Either from atrophy or leuencephalopathy or cerebral radiation necrosis.
4.Changes in neuroimaging characteristics accumulate over time. 5.This complication usually occurs approximately 16 to 18 months after the completion of radiotherapy |
6.Less common delayed complication is focal cerebral necrosis, which usually occurs at the site of tumor.
7.Cerebral radiation necrosis may occur in temporal lobe after head and neck irradiation |
|
|
Name 4 late RT induced vascular complications
|
1)RT induced cavernoma.
2)Aneurysm. 3)Moyamoya disease. 4)Vascular malformation. 5)Mineralazing microangiopathy |
|
|
9.SMART Syndrome:
|
Late Post RT complication:
SMART Syndrome=stroke-like migraine attacks after radiation therapy 1)Headache 2)Focal deficit 3)Seizures. 4)MRI: significant gyral thickening and enhancement that mimic leptomeningeal metastasis. |
|
|
Mechanism of SMART syndrome and late RT complications
|
Mechanism: microvascular damage
|
|
|
Imaging in SMART syndrome
|
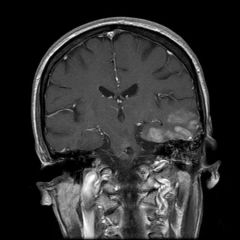
The hallmark of SMART syndrome is prominent and transient gyral enhancement
|
|
|
11.Cerebral radiation necrosis.
1)Fibrinoid necrosis of blood vessels. 2)Vessels hyalinazation. 3)Thrombosis. 4)Telangiectasia |
t
|
|
|
Mx of late RT complications
|
1)resection of cerebral radiation necrosis.
2)RCT proved that Bevacizumab reduce RT necrosis and improve neurological function. 3)Possible role of anticoagulation, hyparic oxygen, combination of Vit E and pentoxifylline. 4)Consider shunt in pt with progressive ventricular enlargement (?NPH). 5)Consider methylphenidate, modafenil or donepezil. |
|
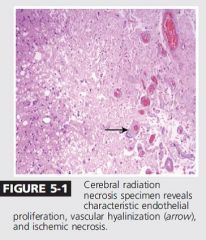
|
vascular hyalinization.
The most accurate Dx is through biopsy |
|
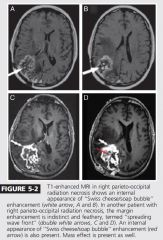
2 imaging features of RT necrosis
|
Swiss chees and spreading wave front
|
|
|
Can RT cause Myelopathy
|
1-Acute,
2-Transient 3-Delayed |
|
|
Acute Myelopathy post RT
|
Ischemic cause
|
|
|
Transient myelopathy
|
about 3 to 6 months after completion of RT (range, 1-30 months).
2)Symptoms often limited to Lhermitte’s sign and paresthesias in the extremities. 3)Transient syndrome with good prognosis 4)pt receiving 4,000 cGy to spinal cord are at higher risk. |
|
|
Onset of delayed myelopathy
|
1)Gradual onset of symptoms often about 14 months (4 months-19 years) after completion of RT:
2)Start with sensory symptoms in the legs (paresthesias and dysesthesias), followed by urinary incontinence and weakness |
|
|
Clinical and imaging features of delayed RT myelopathy
|
May present with brown-Sequard syndrome.
-Most pt experience progressive weakness and some become paraplegic or quadriplegic. -MRI shows spinal cord enlargement and edema, with abnormal intramedullary T2 hyperintensity and sometimes enhancement. 6)With chronic myelopathy and progression, spinal cord may atrophy. 7)Higher risk ass. with higher dosage per fraction and total dose. |
|
|
What are the 2 most common CN affected by RT
|
MC affect are optic and hypoglossal nerves.
|
|
|
post RT Optic neuropathy
|
delayed onset (about 1 Y) of rapidly progressive painless severe vision loss (reduced visual acuity or visual fields, monocular or binocular) in pt receiving radiation to orbits, paraorbital regions, or pituitary region or stereotactic radiosurgery.
2.Possible role for intrvitral bevacizumab. 3.?anotcoagulation??. |
|
|
RT and Brachial plexus:
|
MCC of radiation induced brachial plexus injury is breast Ca.
Presence of horner indicate neoplastic infilteration rather than radiation |
|
|
Subacute Motor Lumbosacral Polyradiculopathy and Lower Motor Neuron Syndrome post RT
|
1)Reversible.
2)TTT: close follow up. |
|
|
Delayed Motor Lumbosacral Polyradiculopathy and Lower Motor Neuron Syndrome post RT
|
Gradual progression of unilateral or bilateral leg weakness with atrophy and fasciculations.
-Predominant involvement of L5,S1 innervated muscel in RT induced lumbosacral plexopathy. -Occurs about 4 months to 1 year after completion of spinal RT. - progression eventually reaches plateau. -MRI plexus is MCC to distinguish RT ve infilterative plexopathy. -Fibrosis can enhance and mimic infilterative process. -Mech: demylination or fibrosis |
|

|
|
|
|

|
|
|
t
|
|
|
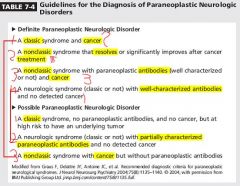
How common is paraneoplastic syndormes
|
1)Much less common than metastatic disease.
2)About 0.01% of cancer patients, but as many as 3% of small cell lung carcinoma |
|
|
Paraneoplastic syndrome time coursse
|
Subacute presentation of typical syndrome is a clue: worsening over weeks to months, but more rapid progression (over a few days) or more insidious course may occur
|
|
|
patients with paraneoplastic syndromes appear to have more favorable oncological outcome than those with similar stage tumors
|
t
|
|
|
CSF and MRI changes in PNS (PARANEOPLASTIC SYNDROME)
|
•Ass e CSF lymphocytic pleocytosis, Hi IgG index, oligoclonal bands.
•BBB is preserved so no enhancement typical seen |
|
|
PNS Investigations
|
•PET / serum markers : CA-125, CA-15.3 and PSA.
•Skeletal survey. •SPEP •BM Bx. •Periodic cancer screening for at least 5 years. •90% discover tumor in 1st year. •Antibiodies present in only 60%, so absence of antibody does not rule out the disease. |
|

|
Limbic encephalitis: HCM, Hu, CRMP, Ma2.
Pancerebellar degeneration PCD CHYT: CRMP, Hu, Yo, Tr |
|
|
|
|
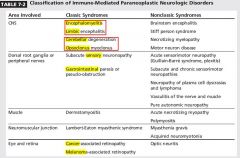
|
Proposed Pathogenesis 1)-Neuroendocrine tumors may express neuronal nuclear, cytoplasmic, or membrane proteins that serve as antigens triggering autoimmune phenomenon.
2)response is both cell-mediated and humoral, although cytotoxic T cells appear to be primary mediators. 3)Once activated, immune system may target normal cells (in nervous system) as well as malignant cells. 4)Often a personal or family history of autoimmunity and higher incidence of other autoantibodies. |
|
|
5)Autoimmune attack is specific, and a more widespread systemic inflammatory response is often lacking; CSF may be normal or only show mildly increased protein level.
6)Antibodies are highly sensitive and specific markers of underlying paraneoplastic syndrome (although may not be highly specific to particular syndromes). |
7)Immunosuppression or removal of tumor in early stage of neurologic syndrome may improve neurologic symptoms.
8)Nonspecific inflammatory changes in CNS: perivascular lymphocytic infiltration (predominantly T cells) and microglial nodules. |
|
|
Malignancies Relatively More Likely To Be Associated With Paraneoplastic Neurologic Syndromes?
|
1)Small cell carcinoma (most commonly, lung)
2)Gynecologic malignancies (breast, ovary, fallopian tube, peritoneum). 3)Hodgkin’s and non-Hodgkin’s lymphoma 4)Testicular cancer 5)Neuroblastoma |
|
|
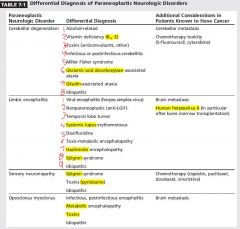
Cerebellar degeneration
1)Subacute onset of gait unsteadiness progressing over weeks to disabling ataxia and severe pancerebellar syndrome. 2)Severe ataxic dysarthria progressing to inability to communicate. 3)Nystagamus (predominant downbeat), vertigo, diplopia, titubation. 4)Symptoms are restricted to cerebellum and related pathways |
5)Imaging: normal early on, cerebellar atrophy in advanced disease
6)Pathology: loss of Purkinje cells. |
|
|
Antibodies associated with Paraneoplastic cerebellar syndrome?
|
Purkinje cell antibody type-1(PCA-1 or anti-Yo, serum or CSF) predicts ovarian cancer (80% of cases) or breast cancer (10% of cases) in women.
8)Positive PCA-1 antibodies warrant exploratory laparotomy if imaging findings are negative. 9)Other antibodies are associated with small cell lung carcinoma (ANNA-1 or anti-Hu, P/Q-type calcium channel, CRMP-5) or 10)lymphoma (anti-Tr) and anti mGluR1. 11)Pure cerebellar findings: anti-Yo and Anti-Tr |
|
|
What is Paraneoplastic encephalomyelitis?
|
Constellation of CNS syndromes, two or more of which may occur concurrently or each may occur in isolation. b. These include limbic encephalitis, brainstem encephalitis, stiff-person syndrome, optic neuritis, transverse myelopathy, motor neuron disease (each of these is discussed separately except for latter two). Affect any part, from CNS, dorsal root ganglia and autonomic nerves.
|
|
|
CSF and MRI in paraneoplastic encephalomyelitis?
|
CSF is always abn: lymphocytic pleocytosis. Hi protein.
Oligoclonal band or Hi IgG index. MRI : Hi Ts signal , no enhancement (anti-Ma2 may enhances). |
|
|
Features of anti Ma2 EM?
|
1)Affect limbic, diencephalon and upper brainstem , hypothalamic,and pituitary (hyperphagia, hypersomnia, narcolepsy, cataplexy, endocrinopathy).
2)Predominant rigidity, hypokinesia, and vertical gaze palsy |
|
|
Features of anti Hu antibody EM?
|
1)Affect lower part of brain stem (medulla and pons).
2)Sensory predominant neuropathy |
|
|
Anti CV2/CRMP5?
|
1) Chorea, uveitis and optic neuritis.
2) Sensorimotor neuropathy. |
|
|
Anti Ri?
|
Opsoclonus myoclonus.
Manidibular and laryngeal spasm. Blepharospasm , trismus. |
|
|
Features of paraneoplastic limbic encephalitis?
|
1)From short-term memory loss to frank dementia.
2)Psychiatric symptoms: personality changes, depression, anxiety psychosis e delusions and hallucination. 3)Sz (complex partial temporal lobe seizures). Excessive sleep. 4)Lesions localized to limbic structures bilaterally (often asymmetric) and also to anteromedial temporal lobes, hippocampus, insula, hypothalamus, amygdale |
|
|
CSFF,EEG, MRI in paraneoplastic limbic encephalitis?
|
5)MRI: enhancing or nonenhancing high T2 signal in mesial temporal lobes (seen best on coronal FLAIR sequences) and in any other areas that may be affected such as brainstem (with concurrent brainstem encephalitis, see below) or hypothalamus (Fig. 16-39).
6)CSF may have transient lymphocytic pleocytosis, increased protein, and increased oligoclonal bands. 7)EEG shows generalized or focal slowing. Bilateral or unilateral temporal lobe epileptic discharges. |
|
|
Most common associated tumor with paraneoplastic limbic encephalitis?
|
small cell lung carcinoma
|
|
|
Most common antibodies associated with parneoplastic limbic encephalitis?
|
a.ANNA- 1/anti-Hu (SCLC),
b.anti-Ma (NSCLC (old men, women), breast, testicular tumors –young man-), c.and anti-Ta (reactive with Ma2 protein, associated with testicular germ cell tumors). |
|
|
1Nonparaneoplastic limbic encephalitis Mb ass e antibodies against ?
|
antibodies against voltage-gated potassium channel, AMPA and GABAb receptors..
|
|
|
Paraneoplastic brainstem encephalitis: (RIM, anti Ri and Ma). 1)With or without cerebellar degeneration
2)Prominent abnormalities of eye movements: diplopia and supranuclear and infranuclear gaze palsy, especiallyvertical gaze palsy. 3)Bulbar involvement: flaccid dysarthria, dysphagia, jaw dystonia. 4)Ptosis and facial weakness. |
5)Spastic quadriparesis, ataxia.
6)Dysregulation of sleep, hypersomnolence. 7)Other paraneoplastic neurologic syndromes may be present concurrently (e.g., limbic encephalitis, cerebellar degeneration). 8)MRI may be normal or show increased signal in affected brainstem |
|
|
What is Opsoclonus and myoclonus?
|
Random high-amplitude,chaotic, arrhythmic, multidirectional saccade without intersaccadic interval and focal or diffuse myoclonus (often involving trunk, limbs, diaphragm, larynx, pharynx).
|
|
|
What is the localization of Opsoclonus and myoclonus?
|
Disinhibition of fastigial nucleus and cerebellum
|
|
|
Name 4 causes of Opsoclonus and myoclonus in children?
|
May be self-limiting disease not associated with tumor and epiphenomenon of underlying viral encephalitis or drug intoxication: good prognosis and response to treatment.
b.When occurs in the setting of neuroblastoma: good prognosis and cancer survival; c.Associated with anti-Hu (ANNA-1) antibodies (neuroblastoma). |
|
|
OMC syndrome in adult?
|
Associated with anti-Hu (ANNA-1 antibodies often associated with small cell lung carcinoma) and anti-Ri (ANNA-2 antibodies, which may be associated with small cell lung carcinoma or breast carcinoma).
b.With or without other concurrent syndromes (including cerebellar degeneration). |
|
|
OMC syndrome Rx and prognosis?
|
When associated with neuroblastoma in children: improves with removal of tumor and with ACTH, corticosteroids.
2)Rituximab and cyclophosphamide. 2) Good recovery in patients without cancer. 3) Worse prognosis for adult-onset paraneoplastic Syndrome |

