![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
163 Cards in this Set
- Front
- Back
|
What 2 theories about electricity evolved from the frog leg experiment?
|
1. Galvani: electricity came from muscle and traveled from nerve to muscle
2. Volta: electricity formed by 2 metals |
|
|
What is an excitable cell?
|
Excitable cells: get some signal => contract
|
|
|
How do ions pass through lipid membrane?
|
can't pass UNLESS via specialized transport mechanism
- pore: ion channel |
|
|
What is an integral protein?
|
transmembrane protein
|
|
|
What is the resting potential of a cell?
|
-60 mV
Reality: -65mv awake; -70 sleeping - influenced by Na and K leak channels |
|
|
What is an AP?
|
short spike of + voltage for signaling
|
|
|
What are the transport mechanism that control things entering and exiting cell?
|
1. active: Consuming energy to move ions against concentration gradient
- Na/K ATPase pump 2. passive: channel mediated diffusion - K leak channel |
|
|
What are the concentration gradients of K and Na?
|
In cell:
K = 155 Na = 12 Out of cell: K = 4 Na = 145 |
|
|
Under what conditions would K+ flow stop?
|
when electrical-chemical equilibrium reached?
- if Na/K ATPase stops |
|
|
What are the driving forces of ion flux?
|
1. electrical (voltage)
2. concentration gradient (chemical) |
|
|
What is happening with K+ at resting potential?
|
- K+ getting actively pumped into cell via K/Na ATPase
- K+ leaking out of cell via leak channel **high [K] in cell |
|
|
How is the membrane potential made?
|
ionic flux generates membrane potential
- uneven charge distribution across membrane from Na/K ATPase - K and Na leak channels |
|
|
How is equilibrium potential calculated?
|
Nernst equation
|
|
|
What are the properties of the ion channels?
|
1. passive (diffusion only)
2. selectivity (particular to given ion - K or Na, etc.) 3. Gating - tells when channel is open |
|
|
What are the 2 types of gating?
|
1. voltage gating: requires particular voltage to occur for appropriate conformational change that would open channel
• can be immediate or delayed response • can be sensitive to voltage for opening or closing 2. ligand gating: requires ligand binding to channel for conformational change to open • ligand gated Na/K channels important • Na – excitatory synapses |
|
|
How is the AP made?
|
K and Na voltage-gated channel
|
|
|
What is the Nerst equation?
What is the simplified Nerst equation? |
EK = RT/ZF 2.3 log Ko/ Ki
E = 59 log [K]o/[K]in |
|
|
When is the Nerst equation used to calculate the membrane potential?
|
at equilibrium
|
|
|
Which ions contribute the most to the resting membrane potential?
|
K and Na
- but Na plays less of a role b/c fewer Na leak channels than K leak channels |
|
|
How does a cell depolarize?
What happens when the cell is more (+) on the inside? |
More Na channels open --> depolarization (excitation)
when more (+) => depolarized – more excited |
|
|
How does a cell hyperpolarize?
What happens when the cell is more (-) on the inside? |
More K channels open --> hyperpolarization (inhibition)
when more (-) => hyperpolarized - more inhibited |
|
|
What does depolarize mean?
What does hyperpolarize mean? |
Depolarize: inside of cell is more positive
Hyperpolarize: inside of cell is more negative |
|
|
What does anesthesia do?
|
Na-leak channel blocking => not feel pain
|
|
|
What is an intracellular recording?
At what level can it measure? |
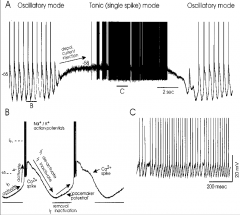
put microelectron into neuron to record
- can measure 20 mV |
|
|
What is an extracellular recording?
At what level can it measure? |
Put wire into neuron/tissue
- 1 mV |
|
|
What are the phases of the AP?
|
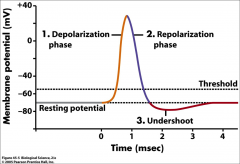
• 1. Initial depolarization
• 2. Repolarization pahse • 3. Undershoot |
|
|
What is the basic idea of an AP?
|
1) V-gated Na channel opens to depolarize membrane close to Na Nernst potential
2) Voltage gated K channel opens to repolarize membrane potential back to resting |
|
|
What is the process of the AP?
|
1. V-gated Na channel opens to depolarize membrane close to Na Nernst potential
2. (+) charge triggers Na voltage gated channel to open 3. Na flows down [] AND electrical gradients 4. Cell depolarizes 5. Na channel rapidly inactivates => prevents further Na movement 6.Voltage gated K channel opens to repolarize membrane potential back to resting 7. (+) cell results in K experiencing high [] AND electrical gradient 8. K leaves cell 9. K efflux hyperpolarizes cell 10. K channels close as potential approaches resting potential |
|
|
What are the 3 states of V-gated Na channels?
|
1. Closed - can be reopened
2. Open 3. Inactive - cannot be reopened |
|
|
Compare the kinetics of voltage gated Na and K channels.
|
V-gated K channels:
• Open slowly by depolarization. • Stay open until membrane repolarize V-gated Na channels: • Open by initial (+) voltage • Close after 2 msec |
|
|
What are the types of refractory periods?
|
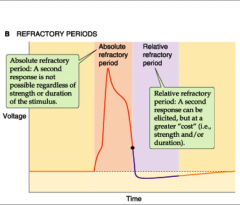
1. Absolute: all Na channels inactive; around time of AP
2. Relative: some Na channels recovered- requires strong signal for another AP; during hyperpolarization |
|
|
What is the purpose of the undershoot?
|
prevent over-exciting
|
|
|
What does the "all-or-none" response mean for AP?
|
If threshold met, AP occurs
- no graded response |
|
|
What is the conduction velocity?
|
speed of propagation of an AP
|
|
|
What does the conduction velocity depend on?
|
internal diameter of axon and internal resistance
|
|
|
What is a benefit of the inactive channels?
|
signal cannot go backwards => signal moves away from origin and goes downstream
|
|
|
What is the effect of a larger diameter?
|
faster conductance velocity b/c lower internal resistance => fewer steps needed => higher conduction velocity
|
|
|
What is the space constant?
|
space constant = square root of (Rm/ Ri )
Ri = 1/ diameter |
|
|
How does membrane resistance affect the conduction velocity?
|
more membrane resistance => faster velocity
|
|
|
How does the internal resistance affect the conduction velocity?
|
less internal resistance => faster velocity
|
|
|
How does capacitance affect AP generation?
|
Capacitance slows down generation of AP
• More capacitance means longer onset time for generating AP – means slower conduction |
|
|
What is capacitance?
|
electrical property of membrane determined by distance (thickness of membrane) and area (shape of axon)
ability of membrane to store electrical charge |
|
|
How does an axon counteract capacitance?
|
myelination
|
|
|
What is the time constant equation?
|
(t) = Rm x Cm
|
|
|
What is the effect of the time constant on conduction velocity?
|
Larger time constant --> slower the velocity
• If onset time Ti for generating AP is longer due to larger membrane capacitance, total velocity is reduced |
|
|
Why does myelin make AP conduction faster?
|
Myelin insulates axon => decreasing capacitance => AP jumps btw Nodes of Ranvier
o Called “Saltatory conductance” |
|
|
Why does the AP jump btw Nodes of Ranvier?
|
Na and K channels [] around Nodes of Ranvier - no myelin sheath
|
|
|
What is contcatin? What is its purpose?
|
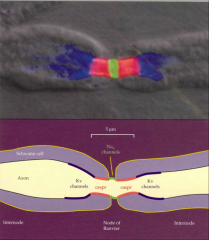
- tight junction proteins in membrane
- seals ends of Schwann cells and Nodes |
|
|
Why does myelination make axons faster
|
reduces capacitance
|
|
|
How do you make an axon faster?
|
1. Saltatory conductance
2. reduced capacitance |
|
|
How would you ID a new channel?
|
look at where the potential equilibrium and reversal potential (where current shifts direction)
|
|
|
Why does the ACh channel have a reversal potential of 0?
|
because it conducts BOTH Na and K
|
|
|
What does a flatter slope on a AP graph mean?
|
needs a lot of stimulus to get a little current
|
|
|
What is a synapse?
|
functional contact between 2 neurons that allows them to communicate with each other via exchange of either chemical or electrical signals
|
|
|
What does a synapse require?
|
2 neurons:
- neuron sending information is called presynaptic - neuron that receives info is called postsynaptic |
|
|
How do pathologies result from synapses?
|
If there are too little or if some are removed
|
|
|
What are the 2 classes of synapses?
|
1. Electric (electro-tonic) synapses: function through electrical signals
- minority (1:10 electrical:chemical synapses) 2. Chemical synapses: function through NTs |
|
|
What does an electrical synapse look like?
|
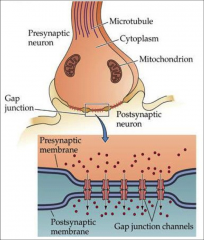
narrow gap junction between pre- & postsynaptic cells
|
|
|
What are the characteristics of electrical synapses? (8)
|
1. faster than chemical synapses
2. permit direct, passive flow of electrical current (no energy requirements) 3. Do not produce inhibitory actions or make long lasting changes in electrical properties of postsynaptic cells 4. no formation of memory (ex: visual system) 5. neuromodulatory 6. induced response < original signal 7. bidirectional impulse transmission 8. synapse essentially tight junctions (3.5 nm distance) |
|
|
What are gap junctions?
What are their characteristics? |
- precisely aligned paired channels that form pores to a junction
- characteristic of electrical synapses - larger than ion channels - allow free diffusion of ions and large molecules (ATP) - tightly regulated transmission of ions initiated in response to AP |
|
|
What are gap junctions made of?
|
made of connexon pore- proteins of tight junctions that span membranes
Presynaptic connexon pair with postsynaptic membrane connexons to form continuous tube |
|
|
What are connexon pores made of?
|
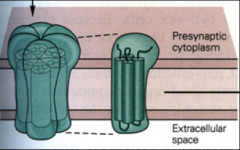
connexin – proteins that cross synapse (go from presynaptic to post-synaptic)
|
|
|
What regulates the opening and closing of gap junctions?
|
open and close in response to Ca
|
|
|
What is the general function of electrical synapses?
|
synchronize electrical activity among populations of neurons
|
|
|
What are the characteristics of the chemical synapse?
|
1. majority of synapses
2. Rely on release of chemical NTs from presynaptic neuron and interaction of NTs with receptors on postsynaptic neuron 3. greater control of strength 4. slower (but still fast) 5. can be inhibitory or excitatory 6. pass info directionally 7. 10x larger distance (than electrical) 8. asymmetric in structure and function |
|
|
What are the 2 junctions btw pre and post synaptic neurons of chemical synapses?
|
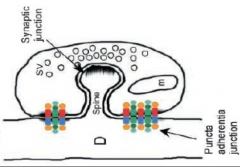
1. Synaptic junctions: site of transmission
- includes: active zone and postsynaptic density 2. Puncta adherentia junctions: mechanical adhesion site/junction - point where membranes in direct contact, but no communication |
|
|
What is the active zone of a chemical synapse?
|
- dark lines btw pre and post synaptic neurons
- aka synaptic grid - all communication occurs here |
|
|
What are presynaptic components of chemical synapses?
|
1. Synaptic vesicles
2. Large dense core vesicles 3. endosomes 4. Smooth ER 5. mitochondria 6. presynaptic dense grid |
|
|
What are characteristics of small vesicles?
|
- small (30-35 nm)
- store all NON-peptide NTs - recycled - fast and spatially precise signaling - do not release contents |
|
|
What regulates the small vesicles?
|
Ca channel
|
|
|
What are the 3 pools of small vesicles?
|
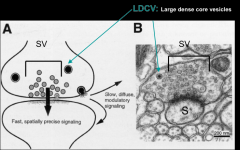
1. readily releasable pool
2. recycling pool 3. reserved pool |
|
|
What are the characteristics of the readily releasable pool?
|
• Docked to presynaptic membrane
• 1st group of vesicles to be released on stimulation (reception of AP) • Small & quickly exhausted - not a lot of them • NTs that are ready to go – closest to synaptic cleft • Used for quick responses • Used when at rest |
|
|
What are the characteristics of the recycling pool?
|
• Proximate/close to cell membrane
• Tend to be recycled at moderate stimulation - taken up by astrocytes • Larger vesicles than readily releasable pool, but it takes longer to become mobilized – requires larger and longer signal to be released into synapse • Used when needing more action – completely normal action |
|
|
When is the readily releasable pool used?
When is the recycling pool used? When is the reserved pool used? |
readily releasable: used at rest, for quick responses
recycling pool: used when needing more action, normal action reserved pool: used when moving or thinking (driving, etc.); Mobilized by intense stimulation & might only occur once other two pools are exhausted |
|
|
what are the characteristics of the reserve pool?
|
- majority of vesicles in neuron
- furthest from membrane - Mobilized by intense stimulation & might only occur once other two pools are exhausted - are present when needed for action |
|
|
How do you distinguish btw types of small vesicles?
|
- size
- distance from membrane |
|
|
How are presynaptic vesicles released?
|
presynaptic vesicles released by exocytosis involving SNARE and Ca
|
|
|
What is SNARE?
|
SNAP receptor
|
|
|
What is SNAP?
|
- soluble NSF attachment protein
• NSF: N-ethylmaleimide sensitive fusion protein - attached to vesicle membrane (v-SNARE) and presynaptic membrane (t-SNARE) |
|
|
What is v-SNARE?
What are the types? |
- proteins that attach to vesicle membrane
- types: synaptobrevin & synaptotagmin |
|
|
What is t-SNARE?
What are the types? |
- proteins attached to presynaptic membranes
- types: syntaxin & SNAP25 |
|
|
How does exocytosis with SNARE work?
|
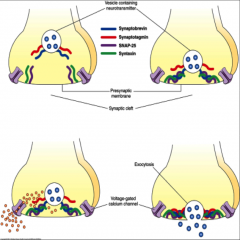
1. Orient vesicle in close proximity to presynaptic membrane
- involves interaction of synaptobrevin with both SNAP-25 and syntaxin (anchor vesicle in place) 2. Influx of Ca due to AP opening voltage-gated Ca channels • Ca interacts with SNARE => synaptotagmin interacts with other 3 SNAREs bringing vesicle closer to presynaptic membrane => fusion and release of NTs |
|
|
How are SNARES made?
|
• Free polyribosomes in presynaptic axon terminal that are locally producing SNAREs (and other proteins required for maturation and recycling of presynaptic vesicles)
|
|
|
What are the characteristics of large dense core vesicles?
|
- 70-200 nm
- contain neuroactive peptides, GF, hormones, amines - abundant in hypothalamic neurons – that contain large amounts of hormones - Synthesized in cell body and antegradely transported - not recycled - Variable numbers, usually fewer than synaptic vesicles - Located distant from active zone - require large, sequential APs for release - Slowest to be released, diffuse, carry out neuromodulatory effects |
|
|
What are endosomes?
|
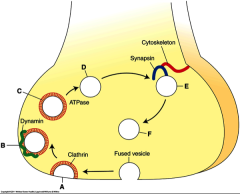
Clathrin (sticky protein; helps endosomes stop moving) coated pits & vesicles
|
|
|
When is clathrin put on endosomes?
|
coated after NT release
|
|
|
What is the purpose of clathrin?
|
allows vesicles to be released from presynaptic membrane
|
|
|
What does on vesicles after they release the NTs?
|
coated with clathrin then dynamin after releasing NTs
|
|
|
When are there a lot of endosomes present in presynaptic neuron?
|
abundant after strong stimulation when vesicles active and releasing contents
• Few endosomes at rest |
|
|
When are endosomes sorted?
Why are they sorted? |
• Segregated from active zones & synaptic vesicle cluster
• Some sorting endosomes destined for retrograde transport, degradation, bulk endocytosis |
|
|
What is the function of the smooth ER in the presynaptic neuron?
|
- regulated intracellular Ca2+ store
- increases efficiency of depolarization and secretion mechanisms – enhances efficiency of depolarization-secretion coupling - **regulates release for large dense core vesicles, but not synaptic vesicle release **more smooth ER in presynaptic than postsynaptic neuron |
|
|
In which neurons does mitochondria accumulate in?
Why? |
• Accumulation of mito in pre but not post synaptic
• Supply ATP for many steps of vesicle cycle: vesicle formation, NT sorting and transport and reuptake and formation |
|
|
What is the presynaptic dense grid?
|
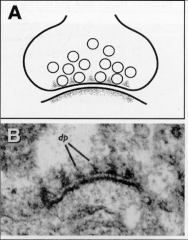
Electron dense, protein region associated with active region of presynaptic membrane
• Refer to electron dense patches (50 nm in diameter) flanked by clear regions • Contains proteins (like t-SNARES) to bring down brevin and vesicle |
|
|
What is the role of presynaptic dense grids?
|
- define NT release sites
- Organizing the docking, release, and recycling of synaptic vesicles - Arranges NTs release sites to be localized with postsynaptic receptor sites |
|
|
What is the synaptic cleft?
|
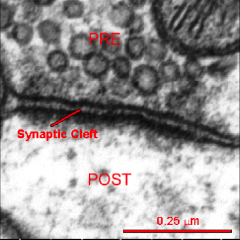
- Fluid-filled space separating pre- & postsynaptic neurons
**distinguishing feature btw electrical and chemical synapses - NTs released into space to interact with receptors on postsynaptic membrane |
|
|
What are the characteristics of transmission across synaptic cleft?
|
- Is a chemical event (as opposed to an electrical one)
- involves release, diffusion, and binding of neurotransmitters. - Ensures unidirectional communication btw neurons - NT swims or floats across cleft |
|
|
What are the postsynaptic components? (4)
What is not there? (2) |
1. postsynaptic density
2. polyribosomes 3. SER 4. endosomal vesicles **NO MITOCHONDRIA OR VESICLES |
|
|
What is the postsynaptic density?
|
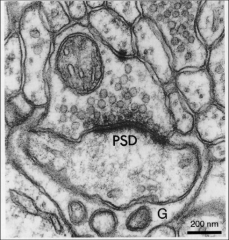
• Analogous to presynaptic dense grid• Composed of protein scaffolds around receptor-signaling microdomains
• Their positioning optimized for linking receptors to 2nd messengers & molecular signaling pathways • coupling of synaptic activity to postsynaptic biochemistry |
|
|
What is the function of the postsynaptic density? (4)
|
1. Organizing postsynaptic receptors
2. positioning 2nd messenger and cell signaling pathways 3. couples synaptic activity with postsynaptic responses through acute events that occur with presence of NTs bound to receptors 4. Molecular organization |
|
|
What is the purpose of having polyribosomes in the postsynaptic neuron?
|
• Polyribosomes: message stays in synapse without protein synthesis until message is needed
|
|
|
What are dendritic spines?
|
small protrusions from dendrites that form concentrated reservoir of signaling and biochemical mechanism
|
|
|
How is a reservoir of signaling molecules formed on dendritic spines?
|
• Reservoir formed due to bottlenecking that limits rate of diffusion of rest of cell
|
|
|
Why are spines important?
|
• Important for activity synchronization in brain
• Form biochemical compartments by limiting diffusion of chemical signals with narrow neck |
|
|
Is there a correlation btw shape of spine and strength of synapse?
|
YES - Strong correlation between the size of spine and the strength of the synapse
CC: abnormal spine #s or shapes associated with variety of nervous system disorders |
|
|
What are the types of dendritic spines
|
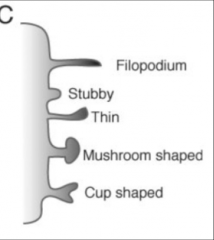
1. small spines
2. large spines 3. stubby 4. thin 5. mushroom shaped 6. cup shaped |
|
|
What are small spines implicated in?
|
plasticity and development
o Implicated in neurological impairment associated with traumatic brain injuries o CC: stroke => lost neurons o CC: Alzheimers => small spines before other sx are noticed |
|
|
What are large spines implicated in?
|
Memory and learning
o Due to larger contact areas that interact with more NT and generate larger functions |
|
|
What are mushroom shaped spines implicated in?
|
Involved in memory formation
- lost => dementia |
|
|
What is the function of filopodium?
|
involved in locating, recognizing appropriate axonal partners and guiding them to the dendrites
|
|
|
What are the 3 types of synapses?
What types of synapses are these? |
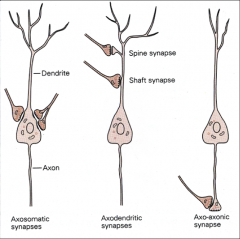
1. axosomatic: axon to cell body
- inhibitory - GABA or glycinergic 2. axodendritic: Axon to dendrite (or shaft synapse or dendritic spine) - Excitatory – with glutamate 3. axo-axonic synapses: axon to terminal ending - involved in presynaptic inhibition |
|
|
What are 2 common morphological types of synapses in the CNS?
What are their characteristics? |
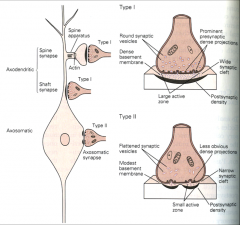
1. Gray type I
• Glutamatergic/excitatory • Typically axondendritic - contact on spines & less commonly contact the shafts of dendrites • Have round synaptic vesicles, large active zone and synaptic cleft, and single continuous postsynaptic density 2.Gray Type II • GABA-ergic/ inhibitory • Axosomatic - Contact the cell body • Have flattened synaptic vesicles, small active zone and synaptic cleft, and multiple perforated postsynaptic densities |
|
|
What is the process of neurotransmission? (10 steps)
|
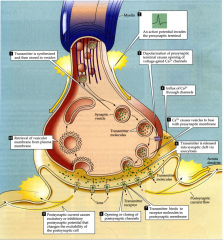
see summary slide
|
|
|
What is implicit memory?
What part of the brain is involved in implicit memory? |
procedural memory of skills and tasks
basal ganglia |
|
|
What is explicit memory?
What part of the brain is involved in explicit memory? |
memory of facts and experiences
hippocampus |
|
|
What are the 4 types of synaptic plasticity?
|
• Synapse formation
• Synapse refinement • Activity dependent plasticity • Synaptic competition |
|
|
How is information stored in hippocampus?
|
circuits are created and turned on/off based on insertion of glutamatergic receptors (NMDA/AMPA)
Stimulus (environment) => insertion of receptors into postsynaptic membrane |
|
|
What is Fragile X syndrome?
What is the mechanism? |
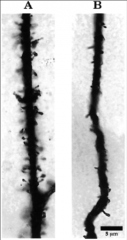
- most common single-gene inherited form of mental retardation
- defect at level of synaptic connectivity - dendritic spines have diff shapes and sizes (B) |
|
|
What is the change in brain morphology with Alzheimer's?
|
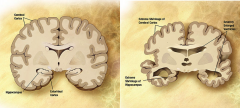
- enlarged ventricles
- extreme shrinkage of cortex and hippocampus **shrinkage of hippocampus => memory deficits |
|
|
What is HEBB's postulate?
|
**When an axon of cell A is near enough to excite cell B and repeatedly and persistently takes part in firing it, some growth process or metabolic change takes place in one or both cells such that A’s efficiency, as one of the cells firing B, is increased
|
|
|
What is the concept of Hebb's postulate?
|
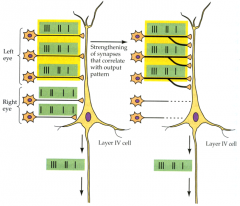
Neurons that fire together, wire together and their connections are preserved
**philosophy underlies LT plasticity |
|
|
What is a Hebbian synapse called?
|
"Coincidence detector"
- neurons encode information by detecting occurrence of temporally close but spatially distributed input signals. Coincidence detectors influence neuronal information processing by reducing temporal jitter, reducing spontaneous activity, and forming associations between separate neural events |
|
|
What 3 components confer Hebbian behavior on a synapse?
|
1. NMDA receptors
2. Back-propagating AP 3. summation of EPSP |
|
|
How does the Hebbian Hypothesis relate to pre and post synaptic neurons?
|
Correlated pre- and postsynaptic activities cause synapse strengthening: stabilization
Uncorrelated pre- and postsynaptic activities cause synapse weakening: elimination |
|
|
How are synapses strengthened?
|
1. High frequency firing
2. Correlated pre and postsynaptic activity |
|
|
What is an "en passant" synapse?
|
synapses that form upstream of boutons
|
|
|
What is a primary function of the hippocampus?
|
memory and learning
|
|
|
What happened to patient HM?
|
Hippocampus was removed => CAN remember childhood memories, CANT make new memories
|
|
|
What are the 3 major synaptic contacts in the hippocampus?
|
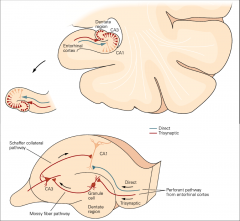
1. Dentate gyrus (DG) = glutamatergic fibers will travel via PERFORANT PATH and synapse onto dendrites of dentate gyrus cells
2. CA3 Region of Hippocampus = DG cells send glutamatergic axon projections in the form of MOSSY FIBERS to this region 3. CA1 region of Hippocampus = = CA3 neurons send axons via the SCAFFER COLLATERAL PATHWAY to this region |
|
|
What are the 3 major pathways of the hippocampus?
|
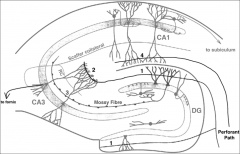
1. Perforant path: from outside of hippocampus to dentate gyrus
2. Mossy fibers 3. Schaffer Collateral Pathway |
|
|
What are the main receptors in the postsynaptic densities of the hippocampus?
What kind of receptors are these? |
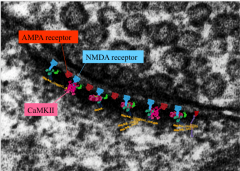
1. AMPA
2. NMDA 3. CaMKII Glutamatergic |
|
|
What are the main signaling complexes in the postsynaptic neurons of the hippocampus?
|
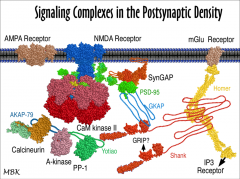
1. Calcineurin
2. CaM Kinase II |
|
|
What are 2 main characteristics of the glutamate receptors?
What are the main glutamate receptors |
- non-selective to cation channels
- permeable to Na, K, Ca AMPA and NMDA |
|
|
Compare AMPA and NMDA receptors.
|
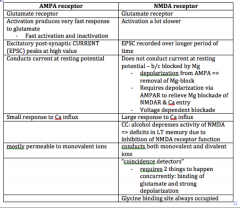
|
|
|
What is the mechanism of activation of CaMKII and autophosphorylation?
|
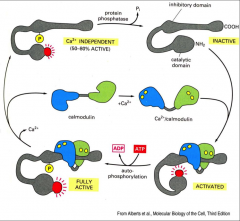
1. CaMKII is activated by the calcium-binding protein calmodulin
2. Autophosphorylation of CaMKII prolongs activation by Ca 3. Activity levels of “Ca2+/calmodulin-dependent protein kinase” change/rise with level of Ca |
|
|
What are the requirements to open the NMDA channel?
|
1. bind glycine and glutamate
2. depolarize membrane to remove Mg block |
|
|
What are the kinetics of the AMPA and NMDA receptors?
|
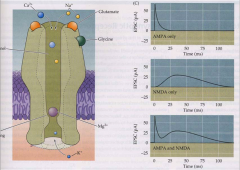
- AMPA: fast rise and subsequent decay (removal of glutamate)
- NMDA: activate and decay under slower time scale |
|
|
What is the effect of alcohol on the NMDA receptors?
|
Alcohol binds with high affinity -- blocks it
|
|
|
What happens when NMDA receptors are active?
|
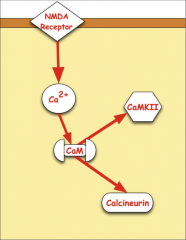
o 1. Ca enters
o 2. Ca binds with calmodulin o 3. Ca MKII and calcineurin pathways activated |
|
|
What is the tetanus test?
What occurs in tetany? |
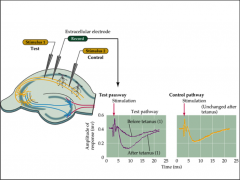
Test: Can stimulate and record Schaffer collateral pathway
- stimulate: release glutamate in a coordinated fashion - if induce high frequency stimulation, tetany occurs PTP (post-tetanic potentiation) caused by large accumulation of Ca2+ in terminal caused by high frequency tetanic stimulation - frequency stimulation leads to increase in amplitude of post-synaptic response over time = LTP => potentiation - potentiated for up to 60 minutes |
|
|
What does LTP depend on?
|
tetany in NMDA receptors
- tetany: high frequency stimulation |
|
|
What does specificity mean?
What does associativity mean? |
**Specificity: if you stimulate particular synapse THAT synapse will be strengthened
**Associativity: when there is weak stimulation in proximity of strong stimulation, weak synapse will strengthen too |
|
|
What is the result of LTD?
|
- counteract LTP
- erase incorrectly learned facts |
|
|
How does LTD work?
|
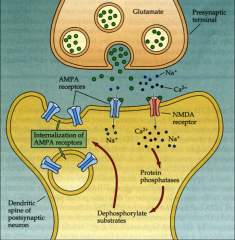
Decrease frequency of firing (<1 Hz) => synaptic efficacy drops
- internalization of AMPA receptors => less strong post-synaptic response |
|
|
Compare LTP and LTD.
|
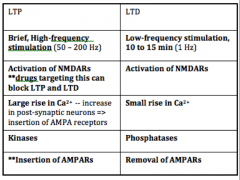
|
|
|
What is plasticity?
When is plasticity the highest in the brain? |
capacity to undergo change in structure and function
Early in life brain is highly plastic - decreases with age |
|
|
What is the Ca hypothesis for control of synaptic plasticity?
|
PTP (post-tetanic potentiation) believed to be caused by a large accumulation of Ca2+ in terminal caused by high frequency tetanic stimulation
|
|
|
What is LTP and LTD triggered by?
|
Ca2+-sensitive signaling machinery located near mouth of NMDA receptor
|
|
|
What happens if you don't have an amygdala?
|
can’t form fear memories
|
|
|
How is the size of synaptic potentials modulated?
|
1. regulating # of vesicles (quanta) released
2. regulating size of current generated by released quantum at postsynaptic membrane |
|
|
Which neuron is responsible for short term modulation?
|
Presynaptic neuron
|
|
|
Which neurons are responsible for long-term plasticity?
|
mechanisms of these forms of modulation are usually both pre- and postsynaptic
|
|
|
What does the postsynaptic neuron control?
|
efficacy of signal
|
|
|
What does synaptic efficacy depend on?
|
FREQUENCY
- LTP: 50-200 Hz - LDP: 1 Hz |
|
|
When does mechanisms for LTP require protein synthesis?
|
- mechanisms for LTP lasting 30 min to a few hr do not require new protein synthesis
- ** mechanisms for LTP lasting longer than a few hr do require protein synthesis. |
|
|
What is the frequency hypothesis?
|
Postsynaptic Ca levels and synaptic plasticity
1. Level and timing of Ca2+ rise in spine determines LTD or LTP. - moderate rise => LTD - high rise => LTP 2. Low frequency synaptic firing (~5 Hz) produces LTD; high frequency synaptic firing (~50 to 100 Hz) produces LTP. 3. same Ca2+ rules underlie “spike-timing-dependent synaptic plasticity (STDP) |
|
|
What are the cellular processes that underlie major changing during LTP and LTD?
|
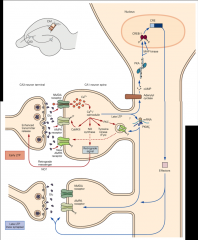
1. Insertion of AMPA receptors into postsynaptic membrane (LTP); or their removal from postsynaptic membrane (LTD).
2.Growth or shrinkage of spine via reshaping of actin cytoskeleton |
|
|
What is CaMKII's role in LTP?
|
1. Ca ion flows through activated NMDA receptor.
2. One of its targets is Ca/calmodulin-regulated Protein Kinase II (CaMKII). 3. CaMKII can phosphorylate subunits of AMPA receptor. • phosphorylated AMPA receptor has a larger current • This is likely one mechanism of relatively short LTP (30 min or so) 4. CaMKII initiates a process that results in addition of new AMPA receptors to the synapse • This process may be developmentally important • It likely also contributes to longer lasting LTP. 5. Helps regulate processes that re-arrange and enlarge cytoskeleton |
|
|
What is calcineurin's role in LTD?
|
1. Ca ion flows through activated NMDA receptor.
2. One of its targets is calcineurin (or protein phosphatase 2B), a Ca2+/CaM-dependent protein phosphatase. 3. Calcineurin regulates inhibitor (Inhibitor 1) of more general protein phosphatase called phosphatase 1. **Inhibition of calcineurin blocks induction of LTD |
|
|
What is the result of LTD?
|
removal of AMPA receptors by endocytosis
|
|
|
How do epigenetic changes in chromatin structure participate in LT memory?
|
Ca signals transduced into nucleus can turn genes on and off
|
|
|
What is the process for gene expression in LTP?
|
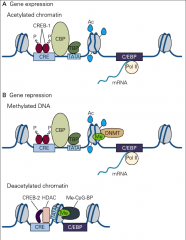
• 1. Phosphorylation of CREB-1
• 2. Recruits CREB Binding Protein (CBP-1) • 3. CBP acetylates lysine(+) resides on histones • 4. Histones release DNA(-) • 5. Allow transcription during late LTP |
|
|
What is the process for gene repression in LTP?
|
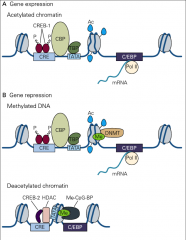
1. LTP changes DNA methyltransferases (DNMT)
2. This recruits methyl-CpG binding proteins (Me-CpG-BP) 3. This recruits histone deacetylases (HDAC), which remove actyl groups 4. This allows CREB-2 binding, which represses transcription |
|
|
What are the molecular bases of LTP at the 3 synapses in the hippocampus?
|
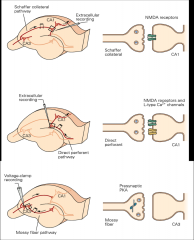
|

