![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
81 Cards in this Set
- Front
- Back
|
What are the top 3 fatal cancers? Men and women?
|
Men:
Lung 30% Prostate 9% Colon & rectum 9% Women: Lung 26% Breast 15% Colon & rectum 9% |
|
|
Cancer is the ____ leading cause of death and accounts for nearly ___ of deaths in the US
|
second
one-quarter |
|
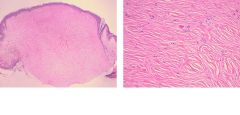
What is this?
|
Fibroma - tumor composed of benign fibroblasts which is producing abundant collagen
|
|
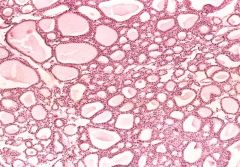
What is this?
|
Benign tumor (adenoma) of the thyroid. Note the normal-looking (well-differentiated) colloid-filled thyroid follicles.
|
|
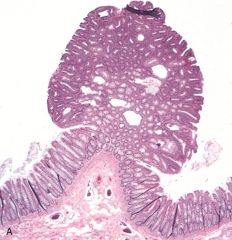
What is this?
|
Colon Polyp - projects into the lumen of the colon. In this case, the polyp is composed of a proliferation of glands with some intervening stroma, and is called a “tubular adenoma.”
|
|
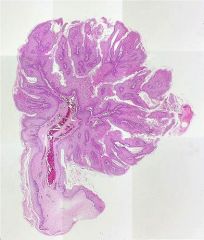
What is this?
|
Squamous papilloma from uvula
|
|
|
Most papillomas are caused by?
|
Human papilloma virus (HPV)
|
|
|
What four malignant tumors end in -oma?
|
Lymphoma
Melanoma Mesothelioma Seminoma |
|
|
Definition of differentiation and anaplasia
|
Differentiation – extent to which the tumor cells resemble the corresponding normal cells
Anaplasia – lack of differentiation |
|
|
Benign tumor characteristics in:
Differentiation Pleomorphism Nuclear morphology Mitoses Polarity Tumor giant cells |
Benign tumor:
Differentiation: Well differentiated Pleomorphism: None to mild Nuclear Morphology: Normal or mild abnormalities Mitoses: few or none Polarity: usually maintained Tumor Gian Cells: None |
|
|
Malignant tumor characteristics in:
Differentiation Pleomorphism Nuclear morphology Mitoses Polarity Tumor giant cells |
Malignant tumor:
Differentiation: Usually at least some loss of differentiation Pleomorphism: Present and often marked Nuclear Morphology: Nuclear enlargement, inc. N/C, hyperchromasia, large nucleoli Mitoses: often numerous, atypical mitotic figures Polarity: usually lost Tumor Giant Cells: occasionally present |
|
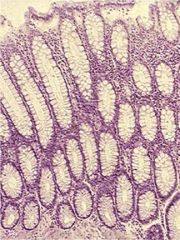
What is this?
|
Normal appearing colonic mucosa
|
|
What is this?
|
Malignant tumor (adenocarcinoma) of the colon
|
|
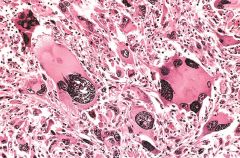
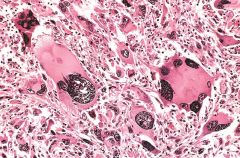
What is this?
|
Anaplastic malignant tumor arising from skeletal muscle (rhabdomyosarcoma). Note the marked cellular and nuclear pleomorphism, hyperchromatic nuclei, and tumor giant cells (arrows).
|
|
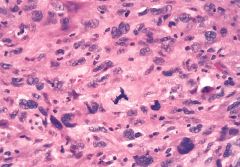
What is this?
|
Anaplastic malignant tumor showing atypical mitotic figure (tripolar mitosis)
Atypical mitotic figures are an even more important indicator of malignancy than the mitotic count. |
|
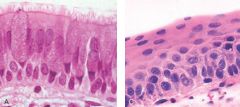
What is this?
|
Left: Histologic section from a bronchus exhibiting the usual respiratory epithelium.
Right: Histologic section from a bronchus in which the respiratory epithelium has been replaced by squamous epithelium, which is referred to as “squamous metaplasia.” |
|
|
Dysplasia primarily occurs in ____ and is characterized by _____
|
epithelium
cells with some anaplastic features; loss of polarity Dysplasia = disordered growth |
|
|
Carcinoma in situ
|
when dysplastic changes involve the full thickness of the epithelium
Considered to be preinvasive cancer; will progress to invasive carcinoma |
|
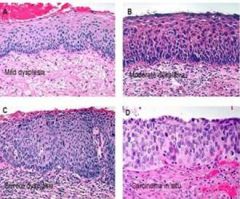
What is this?
|
Histologic sections of cervix exhibiting:
A) mild dysplasia B) moderate dysplasia C) severe dysplasia D) carcinoma in situ. |
|
|
Rate of tumor growth is determined by:
|
-Doubling time of the tumor cells
-Fraction of tumor cells that are in the proliferative pool (growth fraction) -Rate at which cells are shed or die |
|
|
Are growth of tumors associated with shortening of cell cycle time?
|
No
|
|
|
Rate at which tumors grow is ultimately determined by:
|
Excess of cell production over cell loss
|
|
|
When tumors are clinically detectable, are most tumors in the proliferative pool?
|
No, most cells would have undergone reversion to G0, differentiation, or death
|
|
|
Tumor cells with high growth fraction (do/do not) respond well to chemotherapy
|
Do respond well to chemotherapy
Tumors with a low growth fraction are usually refractory Low growth fraction tumors - cells will have to be shift from G0 to cell cycle before chemotherapy works |
|
|
Gross features of benign tumors
|
-Nearly always grow as well-circumscribed masses that remain localized
-Often have a fibrous capsule -Do not invade (infiltrate) into surrounding tissue or metastasize to distant sites -Freely moveable and readily surgically excised |
|
|
Gross features of malignant tumors
|
-Invade (infiltrate) into surrounding tissue
-Poorly circumscribed and fixed to the surrounding tissue -Surgical resection is difficult or impossible -After metastases, invasion is the most reliable feature that differentiates malignant from benign tumors |
|
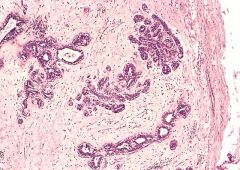
What is this?
|
Histologic section of the fibroadenoma. Note the well-circumscribed appearance of the tumor along with the fibrous capsule, which delimits the tumor from the surrounding adipose tissue.
|
|
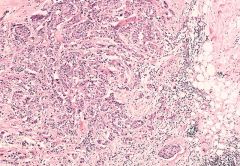
What is this?
|
Histologic section of the invasive carcinoma. Note the haphazardly arranged nests and cords of malignant cells infiltrating through the stroma and contiguous adipose tissue. Also note the absence of any surrounding capsule.
|
|
|
Do all malignant tumors metastasize
|
No
|
|
|
What are the 3 ways of metastatic spread?
|
Seeding of body cavities
Lymphatic spread Hematogenous spread |
|
|
Seeding of body cavities and surfaces occurs when?
This is particularly characteristic of what kind of cancer? |
-occurs when tumor penetrates into a body cavity
-ovarian carcinomas |
|
|
Lymphatic spread is the most common pathway for the initial dissemination of _____ whereas hematogenous spread is the most common pathway for initial dissemination of ____
|
-carcinomas
-sarcomas |
|
|
What is sentinel node Bx?
|
"the first node in a regional lymphatic chain that receives lymph flow from the primary tumor.“
used to assess the presence or absence of metastases in the lymph nodes |
|
|
Lymph node enlargement in proximity to a malignant neoplasm may be due to:
|
Metastatic tumor
Reactive lymphoid hyperplasia |
|
|
What organs are the most frequent sites involved by hematogenous metastases?
|
Liver and lungs
|
|
|
Cancers arising in close proximity to the vertebral column often spread via the ___
|
paravertebral plexus, which leads to vertebral metastases
|
|
|
Hematogenous spread is usually due to the tumor penetrating into the (arteries/veins)
|
veins
|
|
|
What is thought to be the major contributor in the development of most cancers?
|
environmental factors
|
|
|
Alcohol can cause malignancy in what parts of the body?
|
Oral cavity
Upper aerodigestive tract |
|
|
Cigarette smoke can cause malignancy in what parts of the body?
|
Lung
Larynx Pancreas Kidney Bladder upper aerodigestive tract |
|
|
Asbestos can cause what kind of malignancy?
|
Mesothelioma
Lung cancer |
|
|
Benzene can cause what kind of malignancy?
|
Leukemia
Hodgkin lymphoma |
|
|
Vinyl chloride can cause what kind of malignancy?
|
Angiosarcoma of liver
|
|
|
HPV can cause what kind of malignancy?
|
Cervical cancer
|
|
|
Genetic predisposition to cancer is divided into what 3 categories?
|
-Autosomal dominant inherited cancer syndromes
-Defective DNA-Repair syndromes -Familial cancers |
|
|
What type of mutation is usually inherited in "autosomal dominant inherited cancer syndromes"?
What is often associated with this group of people? |
-Usually a point mutation occurring in a single allele of a tumor suppressor gene
-Often associated with a specific "marker phenotype" |
|
|
What are two examples of autosomal dominant inherited cancer syndromes?
What occurs in people with these mutations? |
-Retinoblastoma - 10,000 fold increased risk for retinoblastoma
-Familial adenomatous polyposis - muation of APC tumor suppressor gene; develop multiple adenomatous polyps in colon; 100% affected have colonic adenocarcinoma by age 50 |
|
|
What are 2 examples of defective DNA repair syndromes?
Describe both of them |
-Hereditary nonpolyposis colon cancer (HNPCC)
---Most common cancer predisposition syndrome ---autosomal dominant ---affected often develop multiple colonic adenocarcinomas -Xeroderma pigmentosum ---Autosomal recessive ---patients develop multiple skin cancers |
|
|
What characterizes familial cancers?
|
-early age at onset
-cancer arising in two or more close relatives -sometimes multiple or bilateral tumors |
|
|
How do activated immune cells promote cancer during chronic inflammation?
|
activated immune cells release mediators and reactive 02 species which cause DNA damage along with mediators that promote cell survival, even when DNA is damaged
-proliferation of cells accumulate genetic defects |
|
|
Name the associated neoplasms associated with the following chronic inflammatory conditions:
-Cystitis -Ulcerative colitis -Reflux esophagitis -Gastritis -Lichen sclerosis |
-Bladder carcinoma
-Colorectal carcinoma -Esophageal carcinoma -Gastric carcinoma & mucosa-associated lymphoid tissue (MALT) lymphoma -Vulvar carcinoma |
|
|
What are the four classes of genes that are chief targets for genetic damage when it comes to neoplasia?
|
-proto-oncogenes
-tumor suppressor genes -genes that regulat apoptosis -DNA repair genes |
|
|
Tumor progression most likely results from?
|
multiple mutations that accumulate independently in different cells; subclones of tumor cells
|
|
|
What are oncoproteins?
|
encoded by oncogenes, they resemble normal products of proto-oncogenes, but do not have important internal regulatory elements, which results in autonomous growth and proliferation
|
|
|
What is different in cancer cells when it comes to growth factors?
|
-cancer cells acquire autocrine signaling
-growth factor gene usually not mutated, but overecpressed |
|
|
Tumors may be constantly activated by undergoing what 3 types of changes?
|
-mutations
-gene rearrangements -overexpression (most common) |
|
|
Point mutation in a RET proto-oncogene usually results in?
What about gene rearrangements of RET proto-oncogene? |
-Medullary thyroid carcinoma
-Papillary thyroid carcinoma (RET proto-oncogene encodes for a growth factor receptor) |
|
|
In epidermal growth factor (EGF) ERBB1 is overexpressed in 80% of?
ERBB2 (HER-2/NEU) is over expressed in? |
-squamous cell carcinomas
-breast cancers |
|
|
What is the best example of a signal-transducing oncoprotein?
|
RAS family of proteins (are G proteins)
|
|
|
What is the most common abnormality of proto-oncogenes in human tumors?
|
Point mutation of RAS family genes
|
|
|
Neurofibromitosis is an example of what kind of mutation?
|
RAS (signal-transducing protein) mutation
|
|
|
9;22 translocation is an example of what kind of mutation?
|
non-receptor tyrosine kinases (signal transduction) mutation
|
|
|
What is the most common oncogene involved in mutations in transcription factors?
What kind of tumors is this found in? |
-MYC (c-MYC) oncogene
-many different carcinomas -Burkitt lymphoma (due to chromosomal translocation) |
|
|
What are cyclins and CDKs and what is there role in the cell cycle?
|
CDKs activate by binding to cyclins. They phosphorylate target proteins to drive them through the cell cycle
|
|
|
Overexpression of cyclin D gene is seen in what kind of cancers?
|
-carcinomas of breast, esophagus, and liver
-some lymphomas |
|
|
Amplification of the CDK4 gene occurs in?
|
-melanomas, sarcomas
-glioblastomas |
|
|
Inactivating mutations of CDKI are seen in what kind of cancers? What kind of mutations cause these cancers?
|
-Germline mutation of p16 is seen in 25% of melanoma-prone kindreds
-somatically acquired deletion or inactivation of p16 is seen in pancreatic carcinomas, glioblastomas, and esophageal carcinomas |
|
|
Where are the 2 main checkpoints during cell cycle? What are these checkpoints looking for?
|
-G1/S - DNA damage PRIOR to DNA replication
-G2/M - DNA damage AFTER DNA replication |
|
|
Explain one hit and two hit hypothesis for RB protein causing retinoblastoma
|
-one hit - familial; one is already mutated
-two hit - non-familial; both alleles must undergo somatic mutation |
|
|
How does RB protein regulate G1/S phase?
|
-hypophosphorylated (active) --> binds E2F (blocks entry to S phase) - inactivated by CDKIs
-hyperphosphorylated (inactive) --> releases E2F (allows entry to S phase) - phosphorylated by cyclind-CDK comples |
|
|
Since all cancer cells show dysregulation of the G1/S checkpoint, what are the 4 genes that can be mutated to result in this?
|
-RB
-CDK4 -cyclin D -p16 (last 3 are involved in phosphorylation) |
|
|
Mutations of RB are localized to a region called?
|
RB pocket
|
|
|
What type of viruses can cause transformation of proteins in RB leading to the inability to bind E2F?
|
-polyomavirus large T antigen
-HPV E7 protein |
|
|
What is the most common target for genetic alteration in human tumors?
|
p53 (Guardian of the Genome)
|
|
|
What is the Li-Fraumeni syndrome?
|
-Inherit one mutant p53 allele
-Have a 25-fold greater chance for developing a malignant tumor by age 50 -Develop wide variety of malignant tumors |
|
|
80% of the point mutations on p53 found in human cancers occur where in the protein?
|
in the DNA-binding domain
|
|
|
What can cause p53 to undergo mutation or not function appropriately?
|
-transforming proteins of some viruses can bind to and inactivate p53
-majority of tumors have no p53 mutation, but mutation in another gene causes blocked p53 pathway (ex. MDM2-facilitates degradation of p53; increased p53 is in 33% of sarcomas) |
|
|
What is the role of p53?
|
-cell arrest and repair when damage (GADD45/cyclin dependent kinase inhibitor p21 (CDKN1A)) - if repair fails - apoptosis
-gene repression by activation transcription of mir34 (miRNA family); mir34 repress translation of both proliferative genes and anti-apoptotic genes - results in quiescence, senescence, or apoptosis |
|
|
Malignant neoplasms which have p53 mutations are relatively resistant to what?
|
chemotherapy and radiation - these cause DNA damage, but without p53, cell cannot initiate apoptosis
|
|
|
What is TGF-beta and how does it work?
|
-inhibitor of proliferation
-TGF-beta binds to serine/threonine --> phosphorylation of R-Smads --> binds to co-Smad 4 --> translocated to the nucleus --> increased CDKIs, p21, and p15/INK4B + decrease c-MYC, CDK2, CDK4, and cyclins A and E |
|
|
TGF-beta mutation is commonly seen in what kinds of cancers?
|
-100% of pancreatic cancers
-83% of colon cancers |

