![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
37 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
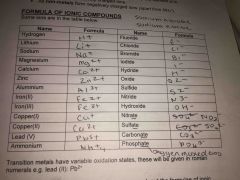
All metals form positively charged ions and non metals forms negative charge. Write the ionic formula for hydrogen, lithium, sodium, magnesium, calcium, zinc, aluminium, iron II, iron III, lead v, ammonium |
H+ , li+, Na+, Mg2+, ca2+, Zn2+, Al3+, fe2+ , fe3+, cu+ cu2+ pb5+ ,Nh+4 |
|
|
|
Transition metals have variable oxidation states these are given in Roman numerical |
|
|
|
|
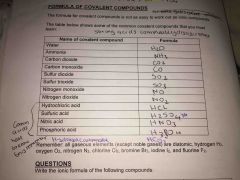
Formula of covalent compounds Water , ammonia, carbon dioxide, carbon monoxide sulfur doixide, sulfur trioxide, nitrogen monoxide, nitrogen dioxide, hudrolochloric acid, sulfur is acid, nitric acid, phosphoric acid, hydrogen carbonate |
|
|
|
|
Write the formula of ionic compound, fluoride, chloride, bromide, iodide, hydride, oxide, sulfide, nitride, hydroxide, nitrate,sulfate,carbonate,phosphate |
F- cl- br- I- H- O2- s2- OH- NO3- SO4 2- CO3 2- PO4 3- |
|
|
|
Common reactions are listed below what is the products of these reactions. 1. Acid + Metal 2. Acid. +. Oxide 3. Acid. +. Metal hydroxide 4. Acid. +. Metal carbonate 5. Acid. +. Hydrogen carbonate 6. Acid. +. Ammonia 7. Metal +. Oxygen 8. Metal carbonate |
1. Salt +. Hydrogen 2. Salt +. water 3. salt +. water 4. salt +. water +. carbon dioxide 5. Salt +. water +. carbon oxide 6. ammonium salt 7. metal oxide 8. metal oxide +. carbon dioxide (on heating) |

|
|
|
What is order of balancing |
Metals Non metals Hydrogen and oxygen
|

|
|
|
How to write ionic equation And what are they useful in |
1 split up aqueous ionic compound (including acids) into separate ions 2. Cancel out the spectator ions that appear on both sides 3. Write final equation they are useful for simplifying precipitation reactions |
|
|
|
When you are writing balanced equations, what do you write when; 1. an element is present 2. If it is an ionic compound 3. If it’s a covalent compound |
1. If it is an element just put its symbol remember to add the two if it’s a diatomic molecule (N,H,O,F,Cl,Br,I) 2. If it is an ionic compound work out it’s formula using the charges on the ions. 3. If it’s a covalent compound you should know this. (NH3, CH4, H2O, HCL) |
|
|
|
What is a Spectator ion, and why do we split the aqueous but not the solid. And write an ionic equation for the following reaction BaCl2(aq) + Na2SO4(aq) ...... BaSO4(s) + 2NaCl(aq) |
Lots of reactions takes place in aqueous solution do not involve all of the ions that are written in the equation. Some species remain in aqueous solution before and after reaction and are known as Spectator ions. In ionic equations spectator ions are left out We split the aqueous because in water aqueous compounds disassociates in to their ions whereas solids stay in their crystal forms. BaSO4(s) |
|
|
|
What is Avogadro’s constant |
The number of particles in one mole of a substance is 6.022 x10to the power of 23 |
|
|
|
What is Avogadro’s constant |
The number of particles in one mole of a substance is 6.022 x10to the power of 23 |
|
|
|
How to work out moles of a substance using Avogadro’s constant |

Number of particles/ number of moles |
|
|
|
What is Avogadro’s constant |
The number of particles in one mole of a substance is 6.022 x10to the power of 23 |
|
|
|
How to work out moles of a substance using Avogadro’s constant |

Number of particles/ number of moles |
|
|
|
How to work out a mass of one atom, molecule, compound and ion. Using Avogadro’s constant ) |

Mass of 1 atom = Ar/L (6.022x 10. 23) You will be asked to give your answers to the appropriate precision. This will be to the same number of significant figures as the data value with the fewest significant figures used in the calculations |
|
|
|
Precision and significant figures |
The data value with the fewest significant figures used in calculations |
|
|
|
Equations for pure solids liquids and gases |
Moles = mass/ mr Units of mass = grams Units of moles = mol Mr must be calculated and quoted to 1dp |
|
|
|
Equations for pure solids liquids and gases |
Moles = mass/ mr Units of mass = grams Units of moles = mol Mr must be calculated and quoted to 1dp |
|
|
|
Equations for gasses |
PV = nRT Units of pressure (p): pa pascals Units for volume (v): m3 Until for temp (T) : K N= moles R= 8.31 Celsius to kelvin + 273 Kpa to pa x 1000 |
Example What volume of hydrogen gas is produced when 6 g of magnesium react with hydrochloric acid at 298 k and 100kpa 1 write balanced equation 2 work out the no. Of moles of mg 3 using the mole ratio work out the no. Of moles of hydrogen produced 4 rearrange the ideal gas equation to find the volume Answer = v = 6 * 10 -3 m3 |
|
|
Equations for solutions |
Concerntration = moles / volume(/1000) Units of concerntration = mildm-3 or M Units of volume = dm3 Units of moles = mol M3 ..... dm3 ........ cm3 X 1000 |
|
|
|
Mole ratios |
When you calculate the moles using moles = mass /mr you can work out the moles of the other species in the reaction using ratio |
|
|
|
Using balanced equations we can calculate the masses of the chemicals reacting or being produced in a reaction |
What mass of calcium oxide would be produced If 30g Of calcium carbonate was decomposed
1. Write a balanced equation for the reaction if not provided 2. Work out the moles of calcium carbonate 3. Using the ratio from the balanced equation to work out the moles of calcium oxide 4. Work out the mass of calcium oxide |

|
|
|
Mole ratios |
When you calculate the moles using moles = mass /mr you can work out the moles of the other species in the reaction using ratio |
|
|
|
Using balanced equations we can calculate the masses of the chemicals reacting or being produced in a reaction |
What mass of calcium oxide would be produced If 30g Of calcium carbonate was decomposed
1. Write a balanced equation for the reaction if not provided 2. Work out the moles of calcium carbonate 3. Using the ratio from the balanced equation to work out the moles of calcium oxide 4. Work out the mass of calcium oxide |

|
|
|
What is a limiting reagent and how do we calculate mass of a substance from it |
In a chemical reaction not all amount of reagent is used. And some may be in access, and some which is not ( this is is know an as the limiting reagent) then we would use the moles of the limiting reagent in the reacting masses calculations. 1. Work out the moles of both the iron and the sulfur 2. Find out which reactants are in excess and which is the limiting reagent 3. Using the moles of the limiting reagent and the ratio from the balanced equation to work out the moles of the substance you are looking for 4. And then work out the mass of the substance using mass =moles x mr |

|
|
|
Density calculations |
Density = mass/ volume Density units = gcm-3 Mass = g Volume = cm-3 Density is usually used with pure liquids but to work out the mass from a measured volume it can be used with solids and gases.
|
|
|
|
Define empirical formula |
An empirical formula is the simplest ratio of atoms of each element in the compound |
|
|
|
Define empirical formula |
An empirical formula is the simplest ratio of atoms of each element in the compound |
|
|
|
What is the general method of working out empirical formula |
1. Divide each mass (or % mass) by the atomic mass of the element 2. For each of the answers from step 1 divide by the smallest one of those numbers 3. Sometimes the number calculated in step 2 will need to be multiplied to give a whole number 4 theses numbers would be the empirical formula |
|
|
|
Define empirical formula |
An empirical formula is the simplest ratio of atoms of each element in the compound |
|
|
|
What is the general method of working out empirical formula |
1. Divide each mass (or % mass) by the atomic mass of the element 2. For each of the answers from step 1 divide by the smallest one of those numbers 3. Sometimes the number calculated in step 2 will need to be multiplied to give a whole number 4 theses numbers would be the empirical formula |
|
|
|
Define molecular How is it calculated |
A molecular formula is the actual number of atoms of each element in the compound You can work it out from the relative molecular mass (mr ) work out how many times the mass of the empirical formula fits into the mr, and find n |
|
|
|
Formula for mass concerntration |
Mass concerntration = mass /volume Units of mass concerntration = gdm-3 Units f mass = g Units of volume = dm-3 To turn the concentration measured moldm-3 into concerntration measured in gdm-3 multiply by the mr of the substance Moldm-3 * mr = gdm -3 The concerntration in gdm -3 is the same as the mass of solute dissolved in 1dm-3
here -3 into consideration measured in the junior -3 |
|
|
|
What is Percentage atom economy and what is it used for |
Atom economy is a measure of what proportion of the products of a reactant is desired and how much is waste. ( the higher the atom economy the less waste product is produced) % atom economy = mr of desired products (mass of useful products) / mr (mass) of all reactants or products |
|
|
|
What is percentage yield And how is it calculated |
% yield tells us about the practical efficiency of the process.
% yield = actual yield( mass of a specified product ) / theoretical yield ( maximum mass of a product) x100 |
Eg CaCO3 ...... CaO+CO2 10g of calcium carbonate was decomposed to give 3.60g of calcium oxide. What is the %yeild of calcium oxide in this reaction 1. Work out the moles of the reactant 2 using the mole ratio from the balance equation work out the theoretical moles of calcium oxide that should have been produced 3 using the theoretical moles of calcium oxide calculate the theoretical maximum mass of calcium oxide 4 work out the % yeild using the equations above Answer is 64.3 % |
|
|
When finding mr dose the big number in front count 2No5 |
No |
|
|
|
What is percentage yeild and how to calculate it |
It’s a measure of the practical efficiency % yeild = mass of specifies product / theoretical mass of a product * 100 |
|

