![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
72 Cards in this Set
- Front
- Back
|
Three basic states of matter
|
-Gas
-Solid -Liquid |
|
|
Categorical Characteristics Gas/Solid/Liquid
|
Volume and shape
Density Compressability Particle Motion Intermolecular Distance |
|
|
Characteristics of a Gas
|
Volume and shape- Expands to fill the volume of its container; consequently, it takes the shape of the container
Density- Low (10^-3g/ml) Compressability- High Particle Motion- Virtually free Intermolecular Distance- Very Large |
|
|
Characteristics of a Liquid
|
Volume and Shape- Has a fixed volume at a given mass and temp; volume principally dependent on its mass and secondarily on temp; it assumes the shape of its container
Density- High (1g/ml) Compressibility- Very low Particle Motion- Molecules or atoms "slide" past each other Intermolecular Distance- Molecules or atoms are close to each other |
|
|
Characteristics of a Solid
|
Volume and Shape- Has fixed volume; volume principally dependent on its mass and secondarily on temp; it has a definite shape
Density- High (1-10g/ml) Compressibility- Virtually incompressible Particle Motion- Vibrate about a fixed position Intermolecular distance- molecules, ions, or atoms are close to each other |
|
|
Solids
|
-comprised of particles that are held close to one another and have limited ability to move around.
-because of the attractive forces, solids behave as a single unit when acted upon external forces -solids may be classified based on the arrangement of the particles (crystalline vs amorphous) |
|
|
Crystalline solid
|
have particles that are ordered
|
|
|
Amorphous
|
have particles that are not ordered
|
|
|
Types of Crystalline solids
|
-Ionic
-Covalent -molecular -metallic |
|
|
Ionic
|
Particles- cations, anions
Forces- electrostatic MP- High Characteristics- Hard, brittle Examples- NaCL, KBr, MgCl2 |
|
|
covalent
|
Particles-atoms
Forces- covalent bonds MP- Highest, sharing = strong bond in regards to crystalline Characteristics- Extremely hard Examples- Diamond, graphite |
|
|
molecular
|
Particles- Molecules
Forces- Various noncovalent interactions MP= Low Characteristics- usually soft Examples- H20, CH3CH2OH Vanderwaals- dipole dipole & London forces |
|
|
Metallic
|
Particles- Metal ions
Forces- Shared electron cloud (metallic bond, formed by orbital overlap) MP- Varies Characteristics- Hard, brittle, soft, malleable (pound flat), ductile (put into wire) Examples- Na, Fe, Cu, Ag, Au |
|
|
Amorphous
|
Particles- Molecules, atoms, or ions
Forces- Covalent or noncovalent MP- Varies Examples- rubber glass |
|
|
Melting points
|
is proportional to the strength of the attactive forces (cohesion) holding the substance in the solid state
-typically the higher the attractive forces, the more energy required to disrupt the solid state, thus the higher MP |
|
|
Liquid
|
is one type of fluid
|
|
|
What is a fluid?
|
-a system of particles loosley held together by their own cohesive forces (intermolecular), or by the restraining forces exerted by the walls of the container
- gases are generally compressible whereas liquids are generally incompressible |
|
|
A "perfect" fluid
|
-offers no resistance to flow, except through its intertia
-gases at low density and higher temps will behave more like perfect fluids -most fluids we will encounter will have an internal friction ("stickiness")that resists flow- viscosity |
|
|
density
|
-amount of mass per unit volume
-symbol rho(p) -p=M/V -units are kg/m^3 |
|
|
conversion unit to meters^3
|
1 liter= 10^-3 m^3
|
|
|
Gases- Definition
|
is one type of fluid
|
|
|
fluid
|
a continous amorphous substance whose molecules move freely past one another and that has the tendency to assume the shape of its container; a liquid or gas
|
|
|
Elastic
|
resist change in shape, snaps back, recoil
|
|
|
shear stress
|
sliding motion, when molecules rub past each other
|
|
|
Gas-the state of matter distinguished from the solid and liquid states by:
|
-relatively low density and viscosity
-relatively great expansion and contraction with changes in pressure and temp -the ability to diffuse (mix) readily -the spontaneous tendency to become distributed uniformly throughout any container |
|
|
Working definition for Gas
|
will completely fill a container or volume in which it is confined
|
|
|
macroscopic properties of gases
|
those properties that reflect the average condition of the gas: moles, volume, density, pressure, and temperature
|
|
|
Gas Laws- Overview
|
Boyle's, Charles', and Gay-Lussac's Laws are actually limited (constrained) versions of the Ideal Gas Law
-the Ideal Gas Law is a combination of Boyle's and Charles' laws |
|
|
Boyle's Law
|
if temp is constant, relates volume and pressure
-volume and pressure are inversely related when T constant -usually states "at fixed temp, volume times pressure is constant" -moles are constant |
|
|
Boyle's Law formula
|
P1V1=P2V2
|
|
|
Applications of Boyle's Law in Anesthesia
|
1. Squeezing BVM increases pressure and decreases volume
2. release of gas from compressed cylinder into atmosphere 3. Body plethysmography 4. Inspiration of gases into lung (increase V of thorax > decreased P) negative pressure breathing > spont vent |
|
|
Charles' Law
|
-sometimes lumped with Gay-Lussac since they are related
-relates volume and temp at fixed pressure -"at fixed pressure that ratio of volume to temp is constant" |
|
|
Charles' Law- formula
|
V1/T1 = V2/T2
-direct relationship |
|
|
Application of Charles Law in Anethesia
|
the inflatable cuff of an ETT or LMA in the autoclave- as temp increases so does volume
|
|
|
Gay-Lussac's Law
|
-often lumped with Charles' law since they are similar
-Relates pressure and temp at fixed volume -Pressure is directly proportional to temp at fixed volume -"at fixed volume the ration of pressure to temp is constant" |
|
|
Gay-Lussac's Law- formula
|
P1/T1=P2/T2
|
|
|
Applications of Gay-Lussac's Law in anesthesia
|
1. When the temp of a closed cylinder increases the pressure increases
2. as the cylinder containing liquid N20 empties, the temp decreases 3. Wood's metal blows when the temp of a cylinder increases substantially |
|
|
Avogardo's Law
|
equal volume of gases at the same temp and pressure, contained the same number of particles, or molecules.
-the number of molecules in a specifc volume of gas is independent of the size or mass of the gas molecules. |
|
|
The ideal gas constant
|
-has the same value for all gases:
P1V1/T1n1=P2V2/T2n2 |
|
|
Molar Volume and Gas Density
|
The volume occupied by 1 mol of any gas is called its molar volume.
-at standard temp and pressure, dry (STPD) the molar volume of any gas is 22.4 L -able to calculate the mass density for any gas by relating the gas's gram-molecular wt to its molar volume, which is always 22.4l/mol @ STPD |
|
|
Ideal Gas Law
|
-Combination of Boyle's, Charles' and Avogardo's law
-PV=nRT |
|
|
R
|
R= 0.0821 L*atm*mol^-1*K^-1
Or R= 8.31 J*mol^-1*K^-1 where J = N*M |
|
|
Pascal conversion
|
1pa = N/M^2
|
|
|
Conversion of atm to Pa
|
1 atm = 1.013 x 10^5 pa
|
|
|
Conversion of atm to torr
|
1 atm = 760 torr
|
|
|
Kinetic theory of gases
|
-describe molecules in a gas as a collection of elastic balls in free, random motion inside of a container
-collisions are perfectly elastic= no energy lost in collision |
|
|
Newtons's Laws
|
the result of each collision of a molecule with the wall of a container creates a tiny force (f) acting on the wall of the container
-the average force exerted in 1 second over a unit area of one wall of the vessel volume (V) is the average force per unit area, which is the definition of pressure (P) |
|
|
Gas triangle memory device
|
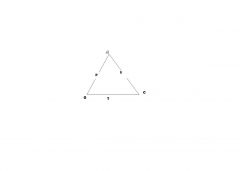
Can these guys possibly be violinists
|
|
|
Graphic summary of Gas Laws
|

The relationship of the gas laws
|
|
|
Summary of gas Pressure
1 |
the pressure of a gas is proportional to the average force per unit area that gas molecules exert on the walls of the conatiner
|
|
|
Summary of gas Pressure
2 |
-the total pressure exerted by all gas molecules in the atmosphere is the barometric pressure, Pb. Units of pressure are millimeter mercury (mmHg), or the kilopascal (kPa)
|
|
|
Pressure conversions
|
-at sealevel Pb (1 atm) = 760mm Hg = 101 kPa
-1 kPa = 7.50mmHg = 7.50 torr = 0.295 inches |
|
|
Summary of gas Pressure
3 |
for low pressures, centimeters of water (cm H20) are often used.
-1mm Hg = 1.35 cm H20, this is derived from the ratio of densities of mercury (13.6gm *cm^-3) and water (1gm*cm^-3) |
|
|
Dalton's Law of Partial Pressures
|
Gas molecules are assumed to act independently, which is a good approximation for low pressure gases
this assumption is the basis for Dalton's law: THE TOTAL PRESSURE OF A GAS MIXTURE (Ptot) IS EQUAL TO THE ALGABRAIC SUM OF THE PARTIAL PRESSURES (TENSIONS) OF ALL THE GASES IN THE MIXTURE. DALTON'S LAW: Ptotal = P1+P2...Pn |
|
|
Dalton's law semantics
|
Chemistry = lower case p for partial and upper case for total
Physiology= pO2, so PO2 |
|
|
Fractional Concentration (percent concentration)
|
-the partial pressure (tension) of a gas is equal to the fractional concentration of a specific gas times the total pressure of the mixture
-relationship derived from Dalton's law |
|
|
Fractional/Percent Concentration formula
|
Pgas= Fgas X Ptotal
-Only holds true for molecules that are "free", that is not chemically combined |
|
|
Applications of Dalton's Laws
|
1. Permits calculation of the % concentration of a gas by dividing the partial pressure of the gas by the total pressure
2. Permits calculation of the partial pressure of a gas by multiplying % contration by the total pressure |
|
|
Vapor Pressure
|
is the partial pressure exerted by gas molecules when there is equilibrium between the liquid and gas phases of the molecules
-if the container is open to air, then the vapor pressure of the molecules is a partial pressure, along with the other gas molecules in the air |
|
|
saturated vapor pressure
|
if the liquid is in a closed container with no other gases, the pressure of the vapor at equilibrium is specifically calleld the saturated vapor pressure
|
|
|
Boiling point of a liquid
|
is the temperature at which the vapor pressure and atmospheric pressure are equal
|
|
|
Important point about boiling point and vapor pressure
|
-BP of a liquid is not constant, the BP is a function of pressure
-the vapor pressure is a functiuon of the temp of the system |
|
|
Gas solubility and Henry's Law
|
-describes the same equilibrium as does saturated vapor pressure, but from the perspective of the fluid rather than the gas.
-at equilibrium, the liquid is saturated with all of the gas molecules that is can hold -Henry's law states that the amt of gas (conc) that can dissolve in a liquid is directly proportional to the partial pressure of the gas above the liquid |
|
|
Henry's Law eqauation
|
Cgas= B(beta) x Pgas
B= solubility coefficient Pgas= partial pressure |
|
|
Solubility coefficient
|
the solubility coeffcient is inversly proportional to temp- increased temp = decreased B
-the concentration is usually expressed per 100ml (deciliter) of fluid, and thus called volume percent |
|
|
B(beta)C02
|
0.067ml CO2 * dl^-1 mmHg @ 37C
|
|
|
B(beta) 02
|
0.003ml O2 *dl^-1 mmHg @ 37C
|
|
|
How much oxygen is dissolved in each deciliter of blood when Po2 = 90mm HG
|
Co2 = Bo2 x Po2
0.003 ml 02/ dl*mm Hg x 90mmHG =0.27 CO2 was 2.6 -o2 does not dissolve in blood very well, good thing we have hemoglobin |
|
|
Absolute Humidity
|
-the mass of water vapor present in a given volume of air
-the values of absolute humidity are usually expressed as mg/L or g/m^3, which are numerically equivalent -the maximum amount of water vapor that can be present in a given volume of aire is a function of the system temperature |
|
|
relative humidity
|
is a ratio of the measured partial pressure of water in the air to the saturated vapor pressure of water, which is determined soley by the temp of the air
-in other words it is the ration of how much water vapor is in the air, compared to the maximum(saturated) amt of h20 that the air could hold at that temp |
|
|
relative humidity formula
|
actual vapor pressure/ saturated vapor pressure
-usually expressed as a percentage |
|
|
Summary of gases
|
-easily compressible
-expand to fill any available volume -have low density -readily diffuse through each other -exert pressure on their containers -behave most ideally at low pressure and high temps |

