![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
146 Cards in this Set
- Front
- Back
|
What is the law of conservation of mass?
|
mass is neither created nor destroyed in a chemical reaction
|
|
|
What are the components of an atom?
|
electron
proton neutron nucleus |
|
|
What is the charge of an atom with 5 protons and 4 neutrons?
|
+5
Atoms are electrically neutral, so the number of protons are balanced by the same number of electrons. In this case, 5(+1) + 4(0) = +5 |
|
|
What is an isotope?
|
have different nuclear properties and masses but the same chemical properties.
Basically, the isotopes have the same number of protons but differ in the number of neutrons. |
|
|
What is the mass number?
|
It is the sum of the protons and neutrons of an element.
It is used to distinguish isotopes from each other. |
|
|
Identify the following groups on the periodic table:
I, II, VII, VIII |
I- alkali metals
II - alkaline earths VII- halogens VIII- noble gases |
|
|
What is significant about all of the members of group VII on the periodic table?
|
VII- halogens
they all have similar properties and they all react with hydrogen to from water soluble compounds. |
|
|
Types of chemical bonds.
|
covalent - sharing electrons
ionic - losing or gaining electrons |
|
|
ionization energy
|
the energy required to remove an electron from the outer shell of an element
|
|
|
What is a miscible liquid?
|
Liquids that dissolve in water.
e.g. alcohols |
|
|
What is an acid?
|
a substance that can donate a hydrogen ion.
|
|
|
What is a base?
|
a substance that can donate a hydroxyl ion.
|
|
|
What is a neutralization reaction?
|
a reaction between an acid and a base that results in a salt and water
|
|
|
what is osmosis?
|
diffusion across a semi-permeable membrane.
relies on osmotic pressure. |
|
|
endothermic reaction
|
reaction in which chemical bonds of the products have more energy than the reactants. Energy is required to make the reaction happen.
ATP is usually the energy source |
|
|
What are catalysts and why are they important?
|
Catalysts are usually enzymes.
They speed up a reaction and lower the activation energy |
|
|
equilibrium constant
|
the point at which reversible reactions have reached an equilibrium between the forward and backward reactions.
KA = reactants/products |
|
|
define a strong acid or base
|
one that is 100% ionized in aqueous solution
|
|
|
what is the equation for pH?
|
pH = -log(H+)
|
|
|
What is the most important buffer in the blood?
|
carbonic acid
H2CO3<->H + HCO3<-> H + CO3 |
|
|
Henderson-Hasselbach equation
|
pH=pKa + log proton acceptor/proton donor
|
|
|
signal transduction
|
the process of converting electrical information into chemical information
|
|
|
4 features of signal transduction
|
specificity
amplification desensitization/adaptation integration |
|
|
3 classes of hormones
(that we focused on in class) |
peptides
steroids catecholamines |
|
|
How do peptide hormones work?
name some examples |
They bind to cell-surface receptors.
e.g. insulin, glucagon, LH, FSH |
|
|
How do steroid hormones work?
name some examples |
enter the cell and bind to receptors in the cytoplasm.
work at the level of transcription e.g. progesterone, estrogen, testosterone |
|
|
How do catecholamines work?
|
act through cell surface receptors to activate 2nd messenger
|
|
|
endocrine secretory pathways
|
endocrine
exocrine autocrine paracrine |
|
|
regulated vs constitutive secretion
|
regulated secretion- hormones are secreted in bursts, so they can be secreted in large amounts over a short period of time.
constitutive secretion - secreting as its synthesized |
|
|
Are catecholamines proteins?
|
NO
|
|
|
How many electrons are in a covalent bond?
|
2 and their spins are opposite
|
|
|
What is the law of conservation of mass?
|
the mass that enters into a chemcial reaction remains unchanged.
Chemical reactions do not create or destroy mass. |
|
|
How do you figure out the mass of an atom?
|
It's the sum of its protons and neutrons.
|
|
|
How do you find the # of electrons of an element b y looking at the periodic table?
|
the # of electrons = the # of protons of an element (the atomic number)
|
|
|
What are valence electrons?
|
Electrons located in the outer-most shell that are available to enter into chemical bonds.
|
|
|
Define atomic number.
|
The number of protons in a given element.
|
|
|
How are the chemical properties of an element derived?
|
chemical properties of an element are related to the number and char. of electrons in the outermost shell.
|
|
|
what is an ion?
|
an atom that has lost or gained an electron. a charged (either + or -) atom
|
|
|
True or False
Elements on the right side of the periodic table lose electrons easily and elements on the left gain them. |
FALSE
Elements on the left lose electrons and elements on the right gain electrons easier. |
|
|
What is meant by an electronegative element?
|
An element on the right side of the periodic table, which means it GAINS electrons easily
|
|
|
True or False
Electronegativity increases from left to right on the periodic table. |
True
|
|
|
What is a polar covalent bond?
|
a covalent bond that unequally shares electrons. The electrons are pulled toward one element more than the other.
|
|
|
Avagadro's number
|
6.02 x 10 to the 23rd
|
|
|
molarity
|
the amount of ions or molecules present in a given volume of solution
|
|
|
1molar solution
|
1M = one mole of a compound in 1 L of solution
one mole = formula weight in grams e.g. formula weight of CH4 = (1x12) + (4x1)=16 one mole = 16 grams |
|
|
True or False
Water molecules are polar. |
TRUE
|
|
|
Define solute and solvent.
|
Solute - component of the solution in the smaller amount; that part dissolved in the solvent.
Solvent - component of the solution in the largest amount and that determines the physical state of the solution |
|
|
Characteristics of a hydrophobic substance
|
insoluble/poorly soluble in water
not charged not polar |
|
|
Define immiscible.
|
liquids that do not dissolve in water.
e.g. oil |
|
|
Fill in the blank.
Gases are (more/less)_______ soluble in water as the temperature rises. |
LESS
Gases are less soluble in water as the temperature rises. |
|
|
Fill in the blank.
Solids that dissolve in water are (more/less)_______ soluble in water as the temperature rises. |
MORE
Solids that dissolve in water are more soluble in water as the temperature rises. |
|
|
What does it mean to have a polar covalent bond?
|
bond where electrons are shared unequally, partial negative charges by the electronegative atom and partial positive charges by the electropositive atom
|
|
|
True or False
All organic compounds containg the carboxyl group are weak acids. |
TRUE
It does not fully dissociate in water. |
|
|
What is pH?
|
the negative log of Hydrogen concentration
|
|
|
What is dissociation?
|
The process of ionization in weak acids and bases.
|
|
|
Bronsted and Lowry theory
|
acids donate protons.
bases accept protons. An acid becomes a base once it donates its proton. |
|
|
Henderson-Hasselbach equation
|
pH=pKa + log(proton acceptor/proton donor)
|
|
|
Define radioactive isotopes and 4 types of radioactive emissions.
|
Radioactive isotopes are elements with unstable nuclei that emit energy as particles or rays.
alpha particle - 2 protons and 2 neutrons (helium nucleus) beta particle - electron from the nucleus gamma ray - high energy electromagnetic wave with no mass or charge positron - positively charged particle the size of an electron |
|
|
Beryllium has atomic number of 4 with an atomic wt of 9. How many neutrons are there?
|
5 neutrons
atomic weight = protons + neutrons atomic number = # of protons |
|
|
True or False
The electrons on the outermost shell are the least energetic and the easiest to lose. |
False
The electrons on the outermost shell are the MOST energetic. |
|
|
CH4 has a formula weight of 16.
What is one mole of CH4? |
16
one mole equals the formula weight in grams. |
|
|
molarity
|
one mole of a compound in one liter solution
|
|
|
Exothermic reaction
|
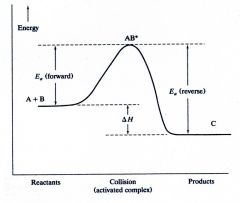
What kind of reaction is this?
|
|
|
Endothermic
|
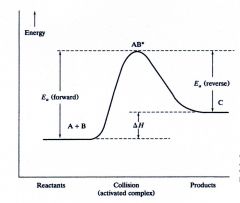
What kind of reaction is this?
|
|
|
What 2 things can speed up an inorganic reaction?
|
increasing the concentration of the reactants
increase the temperature |
|
|
What is a halide?
|
Any element in group VII of the periodic table. Chlorine is the most common.
|
|
|
True or False
All elements with more than 83 protons are radioactive. |
TRUE
|
|
|
True or False
Organic compounds usually have covalent bonds. |
True
|
|
|
True or False
Inorganic compounds usually have covalent bonds. |
False
Inorganic compounds usually have ionic or highly polar bonds. |
|
|
Characteristics of alkanes.
|
consist of hydrogen and carbon
single bond between carbons end in -ane NOT polar NOT charged NOT water soluble ARE oil or lipid soluble |
|
|
structural isomers
|
more than one isomer for a particular chemical formula
|
|
|
4 solubility characteristics of alkanes
|
NOT polar
NOT charged NOT water soluble ARE oil or lipid soluble |
|
|
alcohol
|
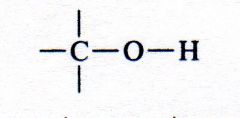
What kind of structure is this?
|
|
|
(alcohol)
methanol |
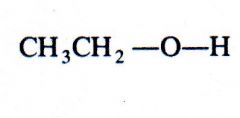
What kind of structure is this?
|
|
|
aldehyde
|
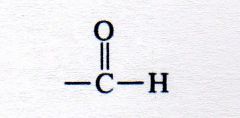
What kind of structure is this?
|
|
|
aldehyde
|
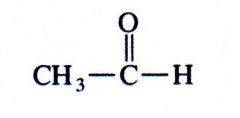
What kind of structure is this?
|
|
|
(alkane)
ethane |
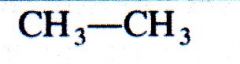
What kind of structure is this?
|
|
|
alkene
|
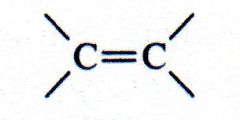
What kind of structure is this?
|
|
|
alkyne
|
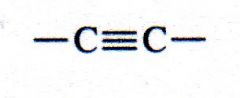
What kind of structure is this?
|
|
|
(alkyne)
acetylene |
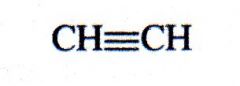
What kind of structure is this?
|
|
|
amide
|
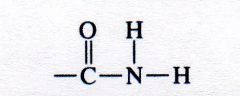
What kind of structure is this?
|
|
|
(amide)
ethylamide |
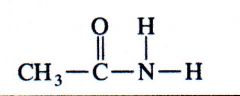
What kind of structure is this?
|
|
|
(amine)
methylamine |
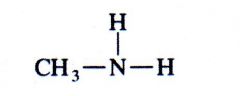
What kind of structure is this?
|
|
|
aromatic
|
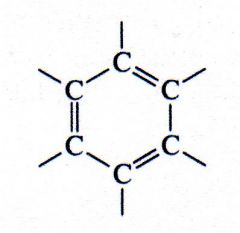
What kind of structure is this?
|
|
|
carboxylic acid
|
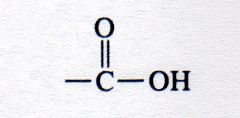
What kind of structure is this?
|
|
|
(carboxylic acid)
ethanoic acid |
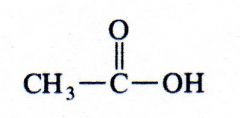
What kind of structure is this?
|
|
|
ester
|
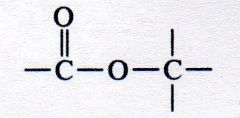
What kind of structure is this?
|
|
|
(ester)
methylethanoate |
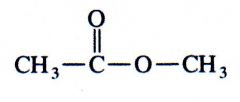
What kind of structure is this?
|
|
|
ether
|
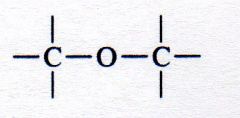
What kind of structure is this?
|
|
|
ether
|
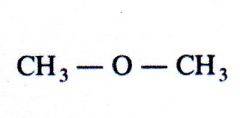
What kind of structure is this?
|
|
|
ketone
|
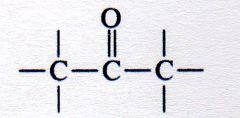
What kind of structure is this?
|
|
|
ketone
|
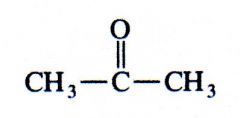
What kind of structure is this?
|
|
|
What are the 4 nucleotides of DNA?
|
A G T C
|
|
|
What is DNA replication?
|
copying of the DNA strand before it divides into daughter strands so each daughter double helix has an original and a newly synthesized strand
|
|
|
In what direction does DNA polymerase read?
|
3 to 5 prime always
|
|
|
What is the 'leading strand' in DNA replication?
|
usually the top strand running 3 to 5 prime
|
|
|
DNA polymerase
|
enzyme that copies the DNA template or leading strand
|
|
|
What kind of bonds are formed between DNA base pairs (A-T; C-G)?
|
Hydrogen bonds
2 Hydrogen bonds between A-T 3 Hydrogen bonds between C-G |
|
|
aliphatic vs. aromatic hydrocarbons
|
aliphatic hydrocarbons lack a benzene ring
aromatic hydrocarbons have a benzene ring as part of their structure |
|
|
solubility of unsaturated hydrocarbons
|
insoluble in water
soluble in oil and fat not polar/uncharged |
|
|
Are alcohols polar or nonpolar?
|
Both.
They are polar at the OH end and nonpolar at the hydrocarbon end. |
|
|
structural difference between aldehydes and ketones.
|
aldehydes have hydrogen attached to the carboxyl group.
ketones have only carbons attached to the carboxyl group |
|
|
What's the difference between a primary, secondary and tertiary amine?
|
The number of hydrogens bound the nitrogen
primary - 2 hydrogens + R group secondary - 1 hydrogen + 2 R groups tertiary - 3 R groups (no hydrogen) |
|
|
stereoisomerism
|
configuration differences with the same connections between the atoms (cis/trans)
|
|
|
optical isomer
|
type of stereoisomer with a chiral carbon. The images are not superimposable.
|
|
|
enantiomer
|
mirror image stereoisomers that are not superimposable
|
|
|
What are the 4 main steps of protein synthesis?
|
DNA replication
DNA transcription RNA translation protein synthesis |
|
|
peptide vs protein
|
peptide-molecules comprised of less than 50 amino acids
protein-molecules comprised of more than 50 amino acids |
|
|
general structure of amino acids
|
carboxylic acid
an amino group a hydrogen atom R group side chain (this defines the uniqueness of the amino acid) |
|
|
in mammals which enantiomers are present, L or D?
|
only L
|
|
|
True or False
Amino acids are symmetric. |
FALSE
Amino acids are assymmetric because they have 4 different constuents (carboxyl, amine, hydrogen and R-group); except glycine which has 2 hydrogens |
|
|
What specifies the polarity of an amino acid?
|
The R group
|
|
|
What is pKa?
|
the dissociation constant.
pH at which half the molecules of an AA are charged and half are uncharged |
|
|
what constitutes a basic AA?
|
R group has a nitrogen
high pKa value (so pH=10-12) |
|
|
what constitutes an acidic AA?
|
R group has a carboxyl group
low pKa negatively charged at physiologic pH |
|
|
phosphorylation
|
The addition of a phosphate group from ATP to an OH group of an AA. Makes the AA negative
|
|
|
Which configuration is more common, cis or trans, and why?
|
Trans because the R groups in the cis configuration tend to repel each other and are too large to be side by side.
|
|
|
What determines the 'primary' protein structure?
|
amino acid sequence
|
|
|
What determines the 'secondary' structure of a protein?
|
alpha helix or beta sheet structure
|
|
|
What determines the 'tertiary' structure of a protein?
|
3-D structure of the folded protein where hydrophobic side chains are internal, hydrophilic or ionized side chains face outward
|
|
|
What is a peptide bond
|
a dehydration reaction b/w 2 amino acids joining the carboxylic acid of one amino acid to the alpha-amino group of another amino acid.
it is a covalent bond. |
|
|
True or false
Cis and trans configurations are common in secondary structure of polypeptide bonds. |
FALSE
Cis configuration is rare because the R-groups repel each other and are too large to be side by side. |
|
|
Primary structure
|
the amino acid sequence of a polypeptide
|
|
|
secondary structure
|
local 3-D folding of a polypeptide chain
|
|
|
cysteine bond
|
disulfide covalent bond between two cysteine amino acids
|
|
|
tertiary structure
|
3-D structure of a polypeptide
|
|
|
What 2 secondary structures are the most observed in naturally occurring polypeptides?
|
alpha-helix
beta sheet |
|
|
alpha helix
|
secondary structure
alpha helix formed by hydrogen bonds with every 4th amino acid down the chain |
|
|
beta sheet
|
secondary structure
side-by-side parallel or ant-parallel alignment of polypeptide chains, each bound by hydrogen bonds |
|
|
True or False
All proteins have a quaternary structure. |
FALSE
|
|
|
quaternary structure
|
noncovalent relationship b/w 2 or more polypeptides to form a multiunit protein
|
|
|
ligand
|
molecules that reversibly bind to other molecules
|
|
|
What is a prosthetic group?
|
a compound that permanently associates with a protein and contributes to its function
e.g. heme prosthetic group |
|
|
oxidation or reduction reaction
ferrous (+2) to ferric (+3) |
oxidation reaction
|
|
|
What is the significance of iron (+3) in hemoglobin?
|
this is methemoglobin. oxygen won't bind to the heme group while iron is in this state.
|
|
|
define: hemoglobin
myoglobin |
hgb-oxygen binding molecule in the blood; consists of 4 protein chains and 4 heme groups
myoglobin - oxygen binding molecule in the skeletal muscle |
|
|
allosteric protein
|
the binding of a ligand to one site affects the binding properties of another site on the same protein
|
|
|
What is the T-state of Hgb?
|
the tense state with no oxygen bound to the heme groups
|
|
|
What is the R-state of Hgb?
|
the relaxed state. As one oxygen binds to a heme group, it results in a conformational change in another heme group increasing its affinity to oxygen until all sites are filled.
Hgb is an allosteric protein! |
|
|
genome
|
the complete set of informtion carried by DNA
|
|
|
intron
|
non translated sequence that interrupts the linear code
|
|
|
exon
|
coding segment for nucleotide sequence
|
|
|
types of RNA
|
rRNA-ribosomal
mRNA-messenger tRNA-transfer |
|
|
rRNA-
|
ribosomal RNA
most abundant type of RNA in a cell carries out translation |
|
|
topoisomerase
|
enzymes that break and rejoin strands of DNA, producing a superhelix
|
|
|
True or False
RNA is linear and single stranded. |
TRUE
|

