![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
142 Cards in this Set
- Front
- Back
|
5 'tasks' of oxygen in the AGM
|
1. oxygen pressure failure alarm
2. fail safe 3. flush valve 4. runs the bellows (drives the ventilator) 5. flowmeter 5. |
|
|
What is considered the high pressure part of the AGM?
|
the tanks (2200 psi)
|
|
|
What is considered the low pressure part of the AGM?
|
components that are distal to the flowmeter to the patient, i.e. common gas outlet, vaporizer, valves
|
|
|
What is considered the intermediate pressure part of the AGM?
|
From the primary regulator to secondary regulator
|
|
|
The primary regulator does what to the pressure coming from the tanks?
|
drops it from 2200 psi to 45 psi
|
|
|
What drives the AGM?
|
oxygen
|
|
|
How much is pipeline pressure?
Tank pressure (before the regulator)? |
50 psi
2200 psi (45psi after the regulator) |
|
|
DISS
|
diameter index safety system
non-interchangeable connection used to connect the pipeline to the AGM |
|
|
What does the check valve do and where is it located?
|
it's located downstream from the pipeline inlet and prevents the reverse flow of gas from the machine to the pipeline
|
|
|
PISS
|
pin index safety system
non-interchangeable connection used to connect the tanks to the AGM; prevents a misconnection of the cylinder to the AGM |
|
|
Why is cylinder pressure less than pipeline pressure?
|
prevents silent depletion of the tank if the tank is inadvertantly left open after checking the pressure
|
|
|
oxygen tank color
|
green
|
|
|
Where is the 2nd stage regulator(s) located on the AGM?
|
they are on all lines before the flowmeter to decrease the pressure from 45 psi to 12-19 psi.
|
|
|
What does the common gas manifold do?
|
mixes all the gases
|
|
|
What is the hypoxic guard?
|
it is a failsafe that does not allow less than 21% oxygen to be delivered to the patient. So, for example, if you increase nitrous, it automatically increases the oxygen content accordingly
|
|
|
What gases flow through the vaporizer?
|
oxygen only
|
|
|
What does the hanger yoke do?
|
it is where the tanks hang.
orients the tank to the AGM provides unidirectional flow ensures a gas-tight seal |
|
|
True or False
The fail safe system prevents hypoxic mixtures of gas. |
FALSE
slide 13 (The Idiots Guide to the Anesthesia Machine lecture) |
|
|
What should you do if you lose oxygen pipeline pressure?
a. open the oxygen cylinder b. disconnect from the pipeline c. ventilate by hand to conserve gas d. A and C e. all of the above |
e. all of the above
Disconnect from the pipeline to ensure that pressure is coming from the cylinder. Ventilate by hand so you eliminate the need for O2 to drive the AGM. |
|
|
How many liters in a full O2 tank?
|
660L
|
|
|
Where is the oxygen flowmeter located on the AGM and why?
|
It is always the farthest downstream in the AGM and closest to the patient so any changes in oxygen flow to the patient will be immediate.
|
|
|
True or False
The hypoxic guard prevents hypoxic breathing mixtures of gases from reaching the patient. |
TRUE
It does permit hypoxic mixtures if the wrong gas is attached to the system, if there is a leak downstream of the flow control valves, if there are defective components on the AGM or if helium is used. |
|
|
True or False
ETT deadspace is higher than mask deadspace. |
FALSE
it is the opposite because the mask has a bubble of air space. |
|
|
Benefits of a closed circuit system include all of the following except:
a. able to conserve respired heat and humidity b. useful for all ages c. resistance is low, less than an ETT d. all of the above are benefits of the system |
d. all of the above are benefits of the system
the disadvantages are increased dead space and possible malfunctions of unidirectional valves |
|
|
2 types of CO2 absorbers
|
soda lime
bara lime |
|
|
Why can't we use sevoflurane with soda lime CO2 absorber?
|
it causes the formation of Compound A, a renal toxic compound
|
|
|
What is the most common mesh size in CO2 absorbers?
|
4 - 8 mesh
|
|
|
True or False
CO2 absorbers always have a color change when they are exhausted and need to be changed. |
FALSE
Color change may not occur as a result of channeling or inactivation of the indicator by UV light. Also, if left alone long enough, a color change of the absorbant will fade. |
|
|
Why is it possible to have exhausted CO2 absorbant without the color change to indicate it?
|
1. channeling
2. inactivation of the indicator by UV light |
|
|
How many liters of gases flow through the I portion of the circuit at any one time?
|
7 liters
|
|
|
What color is the nitrous tank?
|
blue
|
|
|
Why is the scavenger interface so important?
|
It protects the breathing circuit from excessive positive and negative pressures.
|
|
|
Why are descending bellows ventilators becoming obsolete?
|
because it is difficult to recognize patient disconnect from the machine.
|
|
|
4 types of breathing systems
|
open - no rebreathing (ether screen); no mixing of inspired and expired gases
semiopen - those without CO2 absorber semiclosed - those with CO2 absorber closed - rebreathing system |
|
|
What are the Mapleson A & E systems?
|
Mapleson A - 'A' for afferent reservoir system; reservoir bag is on the inspiratory limb; most efficient system for spont. ventilation
Mapleson E - 'E' for efferent reservoir system |
|
|
True or False
Mapleson A’s are inefficient during controlled ventilation (I.e. ambu-bagging) |
TRUE
Mapleson A is good for SPONTANEOUS ventilation because the expiratory valve is farther away from the reservoir bag. Mapleson D is good for controlled ventilation |
|
|
In what order are the Mapleson systems (A,B,C,D) efficient, from most efficient to least efficient?
|
A>D>C>B
"All Dogs Can Bite" |
|
|
List patient/vent factors that influence mixing gases on the Mapleson A system.
|
free gas flow (FGF)
respiratory rate tidal volume expiratory pause CO2 production **we can manipulate FGF |
|
|
Which Mapleson system is best for controlled ventilation?
a. A b. B c. C d. D |
d. D (aka the Bain system)
D is for controlled ventilation. A is for spontaneous ventilation. |
|
|
Which Mapleson system is best for spontaneous ventilation?
a. A b. B c. C d. D |
a. A
A is for spontaneous ventilation. D is for controlled ventilation. |
|
|
List patient/vent factors that influence mixing gases on a Mapleson D system.
|
free gas flow (FGF)
respiratory rate tidal volume pattern of respiration **ALL of them can be manipulated on the D system. |
|
|
What is the average CO2 output for an adult? for a child?
|
adult = 3ml/kg
child = 6ml/kg (higher metabolism) |
|
|
What is the average O2 consumption of an adult?
|
3.5 ml/kg
|
|
|
Systems without CO2 absorbers (open and semiopen) rely on _____ to reduce rebreathing of gas.
|
FGF (free gas flow)
problems with these systems are: wasteful loss of heat loss of moisture |
|
|
What is the APL and what does it do?
|
APL = adjustable pop-off bag
releases gases to the atmosphere or scavenge system provides pressure control in the breathing system |
|
|
True or False
Length of tubing is proportional to amount of dead space. |
FALSE
Length of tubing does not contribute to dead space. |
|
|
list the time constants.
|
1 time constant = 0.63
2 time constants= 0.86 3 time constants= 0.95 4 time constants= 0.98 |
|
|
Of the 4 types of ventilator systems, which one is most used today?
|
semiclosed
|
|
|
The goal in providing anesthesia is to get the patient from Stage ___ to Stage ___, quickly. Stage ___ is an undesirable stage because the patient is _______.
|
I
III II hyperexcitable |
|
|
One time constant equals what percent change in the concentration of a substance toward the total possible change?
|
63%
|
|
|
How many time constants does it take to reach maximal concentration?
|
4
|
|
|
2 time constants = ___%
|
86%
|
|
|
3 time constants = ___%
|
95%
|
|
|
4 time constants = ___%
|
98%
|
|
|
1 time constants = ___%
|
63%
|
|
|
Water is flowing at 2L/min through a pipe with a 15L capacity. Calculate the time constants.
|
time constant = total capacity/flow through system
1Tc = 15/2 = 7.5 min = 63% 2Tc = 15 min = 86% 3Tc = 22.5 min = 95% 4Tc = 30 min = 98% |
|
|
What is the typical volume of an anesthesia system?
|
7L
|
|
|
Which Mapleson system is used to transport patients?
|
Mapleson F
The system's proper function is based on using a gas flow that is twice the pt's minute volume. |
|
|
What should you do if O2 pipeline pressure is lost?
|
Crack the O2 tank
Disconnect the oxygen pipeline supply source at the wall. |
|
|
The oxygen pipeline supply fails and the O2 cylinder gauge shows 1100psi. How long will the tank last if the flow rate is 2L/min?
|
660L/2200psi = x (L)/1100
330L left in the tank with a flow rate of 2L/min = 165 min |
|
|
What is MAC?
|
minimal alveolar concentration
the amount of anesthetic required to provide immobility in 50% of the patient population exposed to surgical stimulation |
|
|
If you increase the flow rate, the time constant will ______.
|
decrease
|
|
|
If you decrease the flow rate, the time constant will ______.
|
increase
|
|
|
What is the partition coefficient?
|
anesthetic solubility
how the gas partitions itself between the blood and the gas |
|
|
With the exception of halothane, how are anesthetic gases excreted?
|
by the lungs, during exhalation
|
|
|
What color is the nitrous oxide tank?
|
blue
|
|
|
What color is the air tank?
|
yellow
|
|
|
What color is the nitrogen tank?
|
black
nitrogen is used to work the instruments in the OR It is NEVER given to the patient. |
|
|
What is nitrogen used for in the OR?
|
to work drills and other instruments
|
|
|
Types of compressed gas? Give examples of each.
|
liquified (CO2, nitrous oxide)
non-liquified (nitrogen, air, helium) |
|
|
Give examples of liquified compressed gases
|
CO2
nitrous oxide |
|
|
Liquified compressed gases are characterized by all of the following except:
a. liquid state at room temperature b. liquid state at a tank pressure greater than 2200psi c. nitrous oxide is an example d. A & C are incorrect |
b. b. liquid state at a tank pressure greater than 2200psi
they are liquid at pressures 25-2500 psi |
|
|
True or False
It is acceptable to start a case with tanks that are 1/2 full. |
FALSE
no laws were violated but the standard of care was violated |
|
|
Which cylinders are larger, E or H?
|
H
|
|
|
How can you determine how much nitrous oxide is in a tank?
|
weigh it
until it is on the verge of being empty, the pressure gage will not change (745 psi). You must weigh it to determine the amount in the tank. |
|
|
Which cylinders are used on the AGM and for transport, E or H?
|
E
|
|
|
2015
|
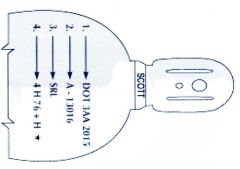
What is the service capacity of this tank?
|
|
|
A-13016
the "*" indicates the 10yr retest interval |
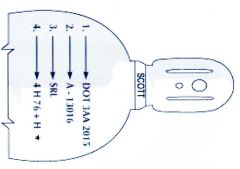
What is the serial number on this tank?
How do you know it qualifies for a 10year hydrostatic retest interval? |
|
|
What is the pin position of the O2 tank?
|
2-5
|
|
|
What is the pin position of the nitrous oxide tank?
|
3-5
|
|
|
What is the capacity of a nitrous oxide tank?
|
1590 L
|
|
|
What is the service pressure of a nitrous oxide tank?
|
745 psi
|
|
|
What is the pin position of the air tank?
|
1-5
|
|
|
What is the capacity of the O2 tank?
|
660L
|
|
|
What is the service pressure of the O2 tank?
|
2200 psi
|
|
|
How long will the O2 tank last if you have 500 psi and you run it at 2L/min?
|
660L/2200psi = X(L)/500psi
x=150L 150L at 2L/min = 75 minutes before the tank is empty |
|
|
You have 745 psi of nitrous oxide in the tank. If you run a flow of 2L/min, how long will the tank last?
|
The only way to figure out how much nitrous is in the tank is to WEIGH IT!!
|
|
|
All the following statements are true except:
a. The pipeline connections to the machine use DISS. b. The diameter and shape of each gas connection is different. c. The PISS system is used on cylinders to ensure easy interchangeability. d. The oxygen connector is 2-5. |
c. The PISS system is used on cylinders to ensure easy interchangeability.
The PISS ensure NON-interchangeability. |
|
|
True or False
Muscle and blood are good conductors of energy. |
TRUE
|
|
|
macro- vs microshock
|
macroshock is an electrical injury as a result of a current applied to the intact skin
microshock is an electrical injury as a result of a current applied to the susceptible tissue lacking the protection of the skin much less current is required to sustain injury in microshock |
|
|
What is LIM?
|
line isolation monitor
electricity in the OR is isolate to limit the amount of surges or outside shocks to the system. LIM is to make sure the leakage current is minimal and that alarms occur when a certain amount of leakage is exceeded. |
|
|
osmotic pressure
|
pressure required to stop osmosis
|
|
|
oncotic pressure
|
pressure exerted by plasma proteins in the capillaries
|
|
|
capillary oncotic pressure
|
24 - 27 mmHg
|
|
|
At what osmolarity do RBC's lyse?
|
<200 mOsm/L
|
|
|
Fick's Law
|
the rate of diffusion of a substance across a unit area (such as a surface or membrane) is directly proportional to the concentration gradient.
It is inversely proportional to the membrane thickness and square root of the molecular weight. |
|
|
Graham's Law
|
the rate of diffusion is inversely proportional to the square root of the molecular weight of the substance.
i.e. big molecules diffuse slower than smaller ones |
|
|
what drives diffusion?
|
entropy
|
|
|
What 3 things are directly proportional to diffusion in Fick's Law?
|
partial pressure gradient
membrane area solubility of gas in membrane |
|
|
Which formula is used to calculate the transmembrane potential difference related to ion diffusion through a semi-permeable?
|
Nernst equation
it takes into account Gibbs' free energy and electromotive force |
|
|
What three factors are important for non-gas diffusion?
|
concentration gradient for un-ionized substances
electrochemical gradient lipid solubility |
|
|
Which will diffuse faster helium or oxygen? Why?
|
Helium
Graham's law --> smaller molecular weight diffuses faster |
|
|
Boyle's Law
|
pressure-volume
at a constant temp, pressure is inversely proportional to volume i.e. if pressure goes up, volume goes down |
|
|
Charles Law
|
temp - volume
at a constant pressure, volume of a gas is directly proportional to the temperature |
|
|
Gay Lussac Law
|
temp - pressure
at a constant volume, the pressure is directly proportional to temp |
|
|
O2 tank in a cold OR is moved to a warm OR. Which law is it?
|
Gay Lussac Law
pressure is directly proportional to temp |
|
|
Avogadro's number and hypothesis
|
6.02 x 10 to the 23rd
one mole is one gram molecular weight at standard temp and pressure |
|
|
General Ideal Gas Law
|
PV=nRT
encompasses Boyle's, Charles', Gay Lussac's Laws and Avogadros' hypothesis |
|
|
Dalton's Law of partial pressures
|
The total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the individual gases.
|
|
|
viscosity
|
the measure of resistance to flow
|
|
|
3 forces associated with liquids
|
pressure
gravity friction |
|
|
With laminar flow, flow is greatest:
a. closest to the wall b. in the center of the tube c. flow is equal throughout the radius of the tube d. none of the above |
b. in the center
|
|
|
Poiseuille's Law
|
1. laminar flow of an incompressible gas/fluid is directly proportional to the fourth power of the radius.
2. flow is directly proportional to pressure 3. flow is inversely proportional to viscosity 4. flow is inversely proportional to length |
|
|
True or False
Flow is directly proportional to pressure |
TRUE
|
|
|
True or False
Flow is directly proportional to viscosity. |
FALSE
It is inversely proportional. the more viscous the fluid the lower the flow. |
|
|
What factors increase the viscosity of blood?
|
decrease temp
increased age increase Hct smoking |
|
|
what is Reynold's number?
|
It measures the propensity for turbulent flow.
>2000 = turbulent flow <2000 = laminar flow |
|
|
When flow is turbulent (viscosity, density) _______ determines flow, not (viscosity, density) _________.
|
density
viscosity viscosity relates to friction whereas density is the weight of a substance. |
|
|
Bernoulli effect
|
the lowering of fluid pressure in regions where the flow velocity is increased (in areas of decreased diameter)
|
|
|
What does the O2 flush valve do?
|
allows O2 to bypass the vaporizer to deliver O2 directly to the patient at 50 psi
can result in barotrauma |
|
|
What does the CO2 absorber do?
|
removes CO2 by absorbing it into the granules
|
|
|
What does the reservoir bag do?
|
stores gas
able to see pt respiring delivers O2 while bagging |
|
|
What does the flowmeter do?
|
allows CRNA to set flow rate of gas which is the amount of O2 that travels through the machine to the patient
|
|
|
What does the vaporizer do?
|
adds anesthetic to the supplied carrier gas
|
|
|
What is the partition coefficient?
|
the ratio of an amount of substance in one phase as opposed to another
i.e. liquid to gas in the lungs as opposed to in the blood useful in anesthesia b/c it indicates the behavior of gases as they enter and leave the body |
|
|
What determines how fast an anesthesia gas works and how fast it wears off?
|
SOLUBILITY
the more insoluble, the faster it works |
|
|
When a pt has a full stomach, how do we go about intubating?
|
awake intubation using topical anesthetics
RSI with antacid admin and cricoid pressure |
|
|
What is the purpose of cricoid pressure?
|
Cricoid pressure is application of downward pressure on the cricoid ring in an effort to prevent passive regurgitation of stomach contents after the patient is unconscious. It will not prevent the muscular activity: vomiting.
|
|
|
What's the difference b/w RSI and regular intubation?
|
application of cricoid pressure
give paralytics and induction agents at the same time use ultra-fast agents to minimize time avoid ventilating to prevent gastric insufflation |
|
|
list 4 induction agents with proper doses.
|
sodium thiopental 3-5 mg/kg IV
propofol 2 mg/kg IV ketamine 2-4 mg/kg IV etomidate 0.2 - 0.3 mg/kg IV midazolam 0.15 -0.4 mg/kg IV |
|
|
list 2 muscle relaxants
|
succinylcholine 1-1.5 mg/kg (depolarizing agent)
rocuronium 1.2 mg/kg (non-depolarizing agent) |
|
|
What is the duration of action of succinylcholine?
|
5-12 minutes
|
|
|
What is the duration of action of rocuronium?
|
60-90 minutes
|
|
|
contraindications to using succinylcholine
|
increased potassium, pts with renal failure, burn pts(succ causes transient potassium release)
use with caution in pts with a head injury b/c succ may cause transient increased ICP |
|
|
How many ancillary people do you need to intubate?
|
1-3
one to hold cricoid pressure one to stabilize neck if injury suspected one to push drugs |
|
|
How do we confirm successful intubation?
|
etCO2
ausculation O2 sat rise condensation on the ETT chest excursion visualization X-ray |
|
|
What does cricoid pressure do?
|
prevents passive reflux of gastric contents
Does NOT prevent active reflux = VOMITING |
|
|
At what age does a pediatric airway become an adult airway?
|
8
|
|
|
How do we figure out the proper ETT size in a child?
|
(age/4)+4
|
|
|
What is the narrowest part in a pediatric airway?
|
cricoid
|
|
|
non-definitive airways?
|
LMA
combitube cricothyrotomy |
|
|
potential complications of a tracheostomy
|
loss of airway
bleeding pneumothorax subcutaneous emphysema cannulation of false passage |

