![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
35 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What is the general structure of skeletal muscle?
|
>Skeletal muscle has a striated appearance when examined under a light microscope
>It consists of multinucleate cells that are bounded by an electrically excitable plasma membrane - Cardiac muscle is also striated >A skeletal muscle cell (1-4 mm long) contains many parallel filaments of myofibrils immersed in the cytosol - The functional unit is the sarcomere - repeats every 2300 nm along the axis. |
|
|
|
Describe the functional unit of skeletal muscle.
|
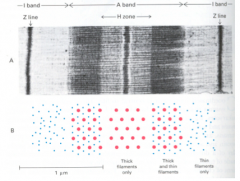
>The functional unit is the sarcomere - repeats every 2300 nm along the axis
- A dark A band and a light I band alternate - Centre of the A band has a less dense H zone and a dark M line at its centre - Centre of the I band has a very dense, narrow Z line >Filaments: - Each thick filament is about 15 nm in diameter (myosin) - Each thin filament is about 9 nm in diameter (actin). - Cross-bridges from thick filaments bridge a 13 nm gap to thin filament |
SARCOMERE
|
|
|
What proportion of skeletal muscle cells are made up of striated muscle proteins?
|
~60%
>33% myosin, 14% actin, 3.5% tropomyosin and troponin (each) - Remaining 40% enzymes |
|
|
|
What is the sliding filament model?
|
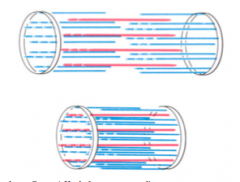
>Muscle shortens by as much as 33% of its original length on contraction
- Lengths of thick and thin filaments do not alter on contraction - Sarcomere shortens because the thick and thin filaments slide past each other during contraction - The force of contraction is generated by an active process of moving one filament past neighbouring filaments of the other type |
SLIDING FILAMENT
|
|
|
How do muscle filaments slide past each other?
|
>ATP-dependent interactions between myosin and actin causes the myosin heads (cross-bridges) to move
- The myosin heads “walks” down the fixed actin filaments - Myosin is a motor protein - a “mechanochemical enzyme” |
|
|
|
What is the primary structure of myosin-II?
|
>Protein of mol. wt. 520,000, and has 6 subunits
- About 10 different myosins are known >Contain two copies of each of three different proteins - Heavy chain - Light chain I - Light chain II |
|
|
|
What makes up the chains of myosin II?
|

3 main components:
- Heavy chain - LMM, S2 and S1 (globlular, others fibrous), S1 contains ATP binding site, an actin binding site and light chain binding sites - Light chain I - regulatory light chain - Light chain II - essential light chain |
MYOSIN
|
|
|
What is the secondary structure of myosin II?
|
>LMM of heavy chain contains 2 alpha helices coiled around each other at 3.5 residues per turn (NOT 3.6)
- Note special packing of hydrophobic aas at the contact point, and charged residues on the outside - 170nm long |
|
|
|
What is the tertiary structure of myosin?
|
>S1 is globular and contains binding sites for ATP and actin
- 17nm long - ATPase site and actin site are on oppositee sides of the N-terminal end of S1 - there is a long neck region to which the RLC and ELC light chain proteins bind |
|
|
|
What is the quaternary structure of Myosin II?
|
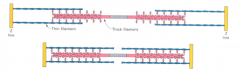
|
MYOSIN II
|
|
|
How is actin structured in striated muscle?
|
>Actin is the most abundant intracellular protein - present in all cells
- Actin is the major protein of thin filaments >It is a monomer of mol. wt. 42,000. It exists as two forms, which are G-actin (globular) and F-actin (fibrous) - G-actin is the monomeric form that prevails at low ionic strength - F-actin exists in thin filaments as a coiled coil, and forms the thin filaments in muscle >When the ionic strength increases to physiological, G-actin polymerises to form F-actin - F-actin is a helical structure that repeats itself after 13 subunits - It has the appearance of two strings of beads wound around each other - Each subunit touches 4 others >ATP binds to actin to accelerate F-actin formation - In all cells, actin filaments provide a lattice that supports the plasma membrane and organises the cytosol - In muscle, it provides the tracks for myosin to move along |
ACTIN
|
|
|
What effect do angel of death mushrooms have on actin?
|
>Angel of Death” mushrooms contain a cyclic peptide toxin called phalloidin which locks F-actin fibres together
|
|
|
|
What occurs in the consensus model for muscle contraction?
|
>Motion is created by coupling ATP binding with actin binding in S1
>Allosteric conformational changes take place in S1 NB. There are two hinges in myosin - between S1 and S2 (for motion) and between S2 and LMM (to assist S1 binding to actin) |
|
|
|
What are the four steps in the consensus model for muscle contraction?
|
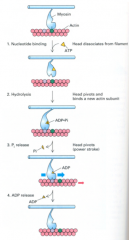
1. ATP binding:
- In the absence of bound ATP, myosin binds tightly - when ATP binds, it closes the ATP cleft in the S1 head and opens the actin binding cleft in the S1 head, so weakening binding to actin - myosin then dissociates from actin 2. ATP hydrolysis - in the free S1 head, ATP is hydrolysed to ADP and Pi - causes the ATP cleft to close, and S1 bends its shape ready to bind to actin again - albeit weakly and in a new position to before 3. Release of phosphate - the release of Pi from myosin causes myosin to bind strongly to actin 4. Release of ADP - the loss of ADP from the ATP cleft causes the S1 head to swivel and straighten up back to its starting position when it can bind tightly to actin - in this 'power stroke' the neck and tail of myosin shifts along the actin filament by 10nm or two actin monomers" |
ACTINMYOSIN BINDING
|
|
|
What is the role of tropomyosin in striated muscle?
|
>There is a 5th stage in muscle contraction which is essential to control this process
- in the absence of ATP myosin does not interact with actin unless the inhibition from two other proteins, tropomyosin and troponin is switched off by a transient rise in the [Ca++] >In resting muscle, calcium is sequestered in the sarcoplasmic reticulum - a Ca-ATPase (SERCA) lowers the [Ca++] in the muscle cytoplams to less than 1uM - a nerve impulse releases calcium to the cytosol and leads to muscle contraction |
|
|
|
What is the role of troponin in striated muscle?
|
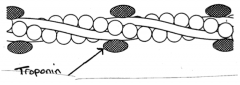
>Attached to the thin filaments is a complex of 3 small regulatory proteins called troponin
>Troponin C binds Ca++ at two sites - when this happens an allosteric conformational change is transmitted to its neighbours troponin I and troponin T - Troponin I binds actin - Troponin T binds tropomyosin and controls the positioning of tropomyosin on the actin filament" |
TROPONIN
|
|
|
In what conditions might a patient be tested for blood troponin?
|
>Suspected heart attack
|
|
|
|
How does striated muscle compare to smooth muscle?
|
>Smooth muscle does not have striations and is under involuntary (unconscious) control of the central nervous system
- filaments are not organised into well ordered sarcomeres, but instead into loose bundles of thick and think filaments at dense bodies in the cytoplams >Contraction occurs as for striated muscle but is now less ordered >In striated and smooth muscle, different regulatory mechanisms control myosin binding to actin - an increase in cytosolic [Ca++] signals contraction in both cases >Calcium phosphorylates the thick filament to allow contraction - this operates more slowly than in striated muscle |
|
|
|
How does the control of muscle contraction differ between striated and smooth muscle?
|
>Smooth muscle contains tropomyosin but not troponin
- hence control of contraction occurs by different mechanisms - unlike striated muscles which are controlled only by nerves |
|
|
|
Which three pathways control muscle contraction in smooth muscle?
|
1. Caldesmon:
- when [Ca] is low, caldesmon forms a complex with tropomyosin and actin and restricts myosin binding to actin 2. Kinase phosporylation: - includes protein kinase C - when caldesmon is phosphorylated, caldesmon cannot bind to the actin bind thin filament and thus cannot inhibit myosin binding to actin 3. Myosin Light Chain Kinase - phosphorylates the regulatory light chain (RLC) of myosin - RLC inhibits actin stimulation of myosin ATPase activity - Ca is needed to activate MLCK, which it does by first binding to calmodulin, then the Ca-calmodulin complex binds to RLC - when RLC is phosphorylated the inhibition is removed NB. Smooth muscle can also be regulated by humoral factors that activate or inhibit contraction e.g. hormones |
|
|
|
What are the properties of calcium in muscle?
|
>Intracellular messenger in many eukaryotic signal transducing pathways
- vision - phosphoinositide cascade - regulation of muscle contraction >Consequently all cells have Ca++ extrusion mechanisms (Ca++-ATPase, Na/Ca exchanger) >[Ca] must be kept very low at 100nM since phosphate esters are abundant while calcium phosphate is very insoluble - hence an abrupt increase in the cytosolic [Ca++] through calcium channels can be used for signalling >Ca can coordinate 6-8 O atoms in asymmetric complexes and can therefore cross-link different segments of a protein and induce large conformational changes |
|
|
|
What are the four roles of calcium in muscle?
|
1. Triggers contraction in striated muscles, by causing the thin filaments to rearrange structurally so that the thick and thin filaments interact with each other
- Myosin ATPase is activated, and filament interdigitation occurs 2. Triggers contraction in smooth muscles, by complexing with calmodulin and activating MLCK to phosphorylate the RLC, causing myosin-actin interactions 3. Ensures sealing f the sarcolemma of striated muscle fibres, so that they do not spontaneously leak Na+ or K+ and depolarise - this is essential for neural control of skeletal muscle contraction NB. tetanus toxin and alkalosis both permeabilise the sarcolemma allowing unprogrammed contraction 4. In less than one second, 5nm Ca++ partially activates phosphorylase kinase in striated muscle - initiates glycogenolysis exactly as work begins and gives muscle partial access to this large fuel store - glycogen is the main initial fuel for all unrehearsed work |
|
|
|
Name an example of a depolarising NMJ blocker.
|
>Suxamethonium
- causes sustained activation of acetylcholine receptors |
|
|
|
What are features of depolarisation block?
|
1. Initial fasciculation (i.e. twitching) as endplate depolarisation causes excitation of muscle fibres
- if pronounced, this is associated with post operative muscle pain 2. Block not relieved and may be enhanced by anticholinesterases 3. Myasthenia gravis patients insensitive to blocking action 4. Action at endplate cause release of K+ from muscle cells, causing hyperkalaemia - normally unimportant, but dangerous either if plasma K+ is already high (e.g. in severe burns, or crush injuries) or if K+ release is enhanced (e.g. diseases or injuries causing denervation) |
|
|
|
How is suxamethonium broken down?
|
>It is hydrolysed rapidly by plasma cholinesterase so action lasts only about 5 minutes
- if plasma cholinesterase activity is low, action can be greatly prolonged, up to several hours - genetic abnormality with low or absent plasma ChE occurs in about 1/3000 - other causes of low plasma ChE include liver disease, malnutrition or use of anticholinesterase drugs |
|
|
|
What two types of cholinesterase are present in tissues?
|
CATALYSES THE THE HYDROLYSIS OF ACH
1. Acetylcholinesterase = true cholinesterase - membrane bound, at all cholinergic synapses - also in red cells (function unknown) - specific for ACh 2. Butyrylcholinesterase (=pseudocholinesterase) - usually a soluble enzyme (sometimes membrane bound) - found in plasma, liver and many other tissues - rather unspecific - hydrolyses butyrylcholine faster than ACh, but many other esters as well including some drugs such as suxamethonium, procaine |
|
|
|
What occurs in genetic variation of butyrylcholinesterase?
|
>BuChE is controlled by an autosomal gene, of which a variant occurs in the normal population
- 1/3000 show 1. greatly reduced rate of hydrolysis of some drugs (especially suxamathonium), clinically important in anaesthesia 2. reduced sensitivity of the enzyme to inhibition by dibucaine - formes the basis of the dibucaine number test carried out on blood samples - normal range is 70-90% inhibition by 10^-4 dibucaine >Heterozygotes have dibucaine number 50-70, do not show abnormal suxamethonium sensitivity >Homozygote have dibucaine number 10-20 and are extremely sensitive to suxamethonium |
|
|
|
What is the mechanism of action of cholinesterase?
|
Enzyme possesses:
1. Esteratic site, containing a reactive serine OH group (like other serine hydrolases) 2. Anionic site, which binds cationic quaternary ammonium group of ACh >enzymic hydrolysis involves a. binding of Ach at the two sites b. transfer of acetyl group to serine OH c. dissociation of choline d. spontaneous hydrolysis of acetylated serine OH >number of ACh molecules hydrolysed at one active site per second is about 10^4, and transmitter ACh released at NMJ is normally hydrolysed in less than one msec |
|
|
|
How do cholinesterase inhibitors work?
|
>They possess:
1. Esteratic site, containing a reactive serine OH group (like other serine hydrolases) 2. Anionic site, which binds the cationic quaternary ammonium group of ACh >Hydrolysis involves - binding of Ach at the two sites - transfer of acetyl group to serine - dissociation of choline - spontaneous hydrolysis of acetylated serine OH >Number of ACh molecules hydrolysed at one active site per second is about 10^4 and transmitter released at NMJ is normally hydrolysed in less than one msec |
|
|
|
How are cholinesterase inhibitors classified?
|
>According to duration of action
1. Short acting anticholinesterases e.g. edrophonium, possess a quaternary ammonium group, but no group complementary to the the esteratic site - act competitively, by binding to the anionic site 2. Medium or long acting reversible AChEs e.g. neostigmine, physostigmine - most important type clinically (e.g. MG), bind to both sites and form an ester bond with serine OH of esteratic site - drug molecule hydrolysed in the process - spontaneous hydrolysis restoring OH is much slower than splitting of acetylated enzyme, so enzyme molecule is inactivated for several minutes by each drug molecule split 3. Long acting irreversible anticholinesterases e.g. dyflos (+DFP), parathion, ecothiopate - organophosphate compounds which phosphorylate the serine OH - hydrolysis of phosphorylated enzyme is negligibly slow, and recovery normally requires synthesis of new enzyme - with a few compounds (e.g. ecothiopate) hydrolysis occurs in a few hours, so action is briefer - most organophosphates have no quaternay group and inhibit many serine enzymes besides ChE |
|
|
|
What are the peripheral effects of of AChE inhibitors?
|
1. Parasympathetic effects - salivation, sweating, increased GI motility (hence vomiting, colic, diarrhoea, bronchoconstriction, bradycardia, pupillary constriction (leading to a fall in intraocular pressure)
2. NMJ - facilitation of transmission, repetitive firing at normal junctions, restoration of transmission at junctions blocked by competitive blocking agents or in myasthenia gravis - with high doses e.g. insecticide poisoning, circulating ACh builds up causing depolarising neuromuscular block |
|
|
|
What are the central effects of AChE inhibitors?
|
>Mainly excitatory, leading to agitation and convulsions, followed by respiratory depression
|
|
|
|
What are the main uses of AChE inhibitors?
|
1. Treatment of glaucoma - physostigmine or organophosphate
2. MG - neostigmine/pyridostigmine as they do not reach the brain (atropine may be needed to control the parasympathetic effects) 3. Reversal of competitive neuromuscular block following anaesthesia 4. Organophosphates are widely used as insecticides, and poisoning is quite common among agricultural workers and gardeners |
|
|
|
What is the key adverse effect of AChE inhibitors?
|
>All AChE have acute toxicity, resulting from central and peripheral actions listed above
- in addition, many organophosphates have an important chronic toxic effect DEMYELINATION - this leads to paralysis and sensory loss, probably due to inactivation of an enzyme involved in myelin synthesis |
|
|
|
What can -oxime compounds do to AChE inhibitors?
|
>Reactivates ChE following phosphorylation
- used in treatment of organophosphate poisoning - combine with the anionic site and entice the phosphate group from the serine OH to the oxime OH group e.g. pralidoxime |
|

