![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
46 Cards in this Set
- Front
- Back
|
What is the molecular action of lipophilic hormones?
|
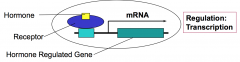
The molecular action of lipophilic hormones (e.g. steroids) is to control gene expression. Lipophilic hormones diffuse through the plasma membrane and bind to intracellular receptors that are transcriptional regulators.
|
|
|
What is the molecular action of hydrophilic hormones?
|
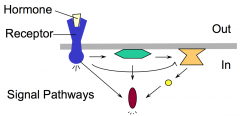
The molecular action of hydrophilic hormones is ultimately to control activities of cellular proteins, including enzymes, transcription factors, cytoskeletal proteins. Hydrophilic hormones bind to extracellular domain of cell surface receptors that span the membrane. This extracellular binding event "activates" the cytoplasmic domain of the receptor and results in the stimulation of one or more intracellular signaling pathways. Signaling pathways are comprised of several different classes of molecules that function in a relay.
|
|
|
What classes of proteins typically regulate activation states of intracellular signal relay components in response to a hydrophilic hormone binding to its cell-surface receptor?
|
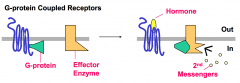
G Proteins and protein kinases typically regulate activation states of intracellular signal relay components in response to a hydrophilic hormone binding to its cell-surface receptor.
|
|
|
What mathematical relationship of the key equilibrium and catalytic rate constants governs the amount of G-protein in the active state?
|
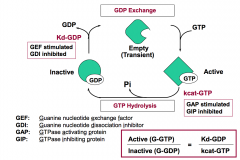
(Active G-protein)/(Inactive G-protein) = (Kd-GDP)/(kcat-GTP)
|
|
|
What classes of regulatory proteins function to increase the fraction of G-protein that is in the “active state”? What step in the G-protein cycle does each of these regulate? What is the mechanism underlying their regulatory effect?
|
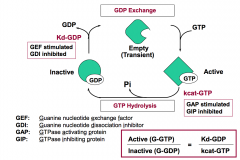
Guanine nucleotide exchange factor (GEF) affects the release of GDP (Kd-GDP). It increases the rate of GDP exchange for GTP and promotes G-GTP formation.
GTPase inhibiting protein (GIP) acts on the hydrolysis of GTP (kcat-GTP). It decreases the rate of GTP hydrolysis and traps G protein in the bound state. |
|
|
What mechanisms are commonly used to inhibit activity of protein kinases in the cell? Which of these is relieved by the action of second messengers?
|
Mechanisms: Negative phosphorylation that serves to stabilize an inactive conformation of the protein kinase. Binding of negative regulatory subunits (or domains) to the catalytic subunit (or domain) of a protein kinase. This binding interaction inhibits activity of the kinase catalytic subunit (or domain).
Reverse inhibition is relieved by the action of second messengers. The second messenger binds to the negative regulatory subunit (or domain) of a protein kinase. Binding weakens the interaction of the negative subunit (or domain) with the catalytic subunit (or domain). |
|
|
What are producer cells?
|
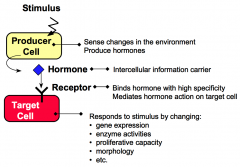
Producer cells sense specific stimuli from the external environment and produce hormones in response to these stimuli.
|
|
|
What are target cells?
|
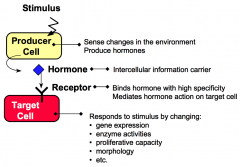
Target cells have receptors for hormones. Hormone binding to receptor causes the target cell to respond by changing cellular functions.
|
|
|
What are hormones?
|
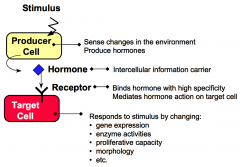
Hormones are intercellular information carriers between sensor/producer cells that perceive changes in the environment and target cells that respond to the change. Hormones coordinate activities of different cells in a multicellular organism.
|
|
|
What are receptors?
|
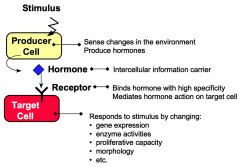
Receptors are proteins that bind hormones with high specificity and affinity.
|
|
|
What are responses?
|
Responses are changes in cellular processes including gene expression, enzyme activities, proliferative capacity, secretory properties, and morphology.
|
|
|
What are the three classifications of hormones, when classified by radius of action?
|
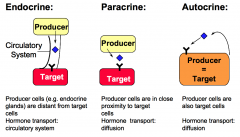
1. Endocrine hormones are produced by endocrine glands and transported by the circulatory system to the target cells.
2. Paracrine Hormones are produced by cells that are in close proximity to the target cells. 3. Autocrine Hormones are produced by the cells that are also the target of action. |
|
|
Which second messenger is catalyzed by the effector Adenylate cyclase?
|
cyclic AMP (cAMP) is the second messenger for Adenylate cyclase.
|
|
|
Which second messenger is catalyzed by the effector Guanylate cyclase?
|
cyclic GMP (cGMP) is the second messenger for Guanylate cyclase.
|
|
|
Which second messenger is catalyzed by the effector Phospholipase C?
|
The Phosphatidylinositol system is comprised of:
Diacylglycerol (DAG) Inositol 1,4,5-triphosphate (IP3) These are the second messengers of the effector Phospholipase C. |
|
|
Which second messenger is catalyzed by the effector Phosphatidylinositol-3-kinase?
|
Phosphatidylinositol(3,4,5)P (PIP3) is the second messenger catalyzed by the effector Phosphatidylinositol-3-kinase.
|
|
|
What is the second messenger of the ion channel system?
|
Calcium is the second messenger of the ion channel system.
|
|
|
What is the role of protein kinases?
|
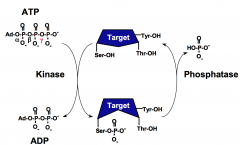
Protein kinases transfer the γPO4 from ATP to the hydroxyl group of serine, threonine, or tyrosine residues of proteins.
The phosphorylation state of a protein affects its biological function through changing its activity, protein associations, stability, or cellular localization. |
|
|
What are the three types of protein kinases?
|
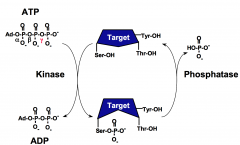
Protein kinases are classified as one of three types:
1. Serine/Threonine Kinase 2. Tyrosine Kinase 3. Dual specificity kinase (both 1 and 2) |
|
|
What is the role of protein phosphatases?
|
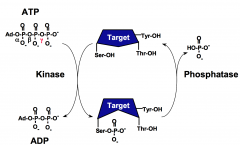
Protein phosphatases reverse the action of protein kinases by hydrolyzing phospho-serine, phospho-threonine, or phospho-tyrosine residues and restoring the side chains to the free hydroxyl form.
The phosphorylation state of a protein affects its biological function through changing its activity, protein associations, stability, or cellular localization. |
|
|
What are the four conserved structural features of the protein kinase catalytic domains?
|
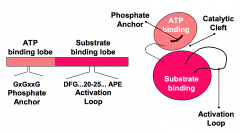
1. ATP binding lobe
2. Substrate binding lobe 3. Catalytic center 4. Activation loop |
|
|
What is the function of the activation loop of the protein kinase catalytic domain?
|
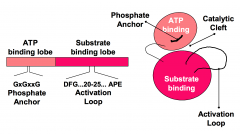
Conformation and phosphorylation state of the Activation Loop determines the spatial relationship of the small and large lobes and the orientation of residues in the cleft that are important for catalysis.
|
|
|
How do regulatory domains or subunits positively regulate protein kinase activity?
|
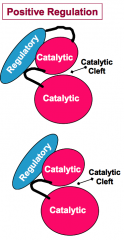
Regulatory domains or subunits can positively regulate protein kinase activity by inducing the active conformation of the kinase catalytic cleft.
|
|
|
What is the difference between a regulatory domain and a regulatory subunit?
|
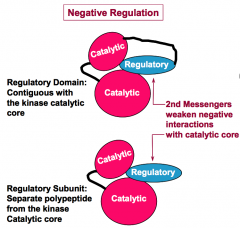
A regulatory domain is a part of the same polypeptide as the protein kinase catalytic core. A regulatory subunit is a separate polypetide from that of the protein kinase catalytic core.
|
|
|
How do regulatory domains or subunits negatively regulate protein kinase activity?
|
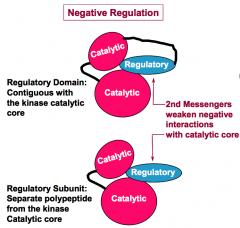
Negative regulation involves protein interactions that disfavor the active conformation of the kinase catalytic core and/or interfere with substrate binding. (This regulation is often reversed by the action of second messengers.)
|
|
|
How does one protein kinase positively regulate the activity of another protein kinase?
|
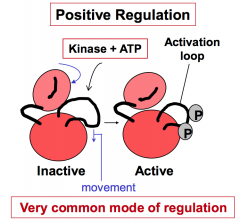
Positive regulation by covalent addition of phosphate(s) promotes active conformation of the kinase (e.g. addition of phosphates to the activation loop). This is a very common mode of regulation.
|
|
|
How does one protein kinase negatively regulate another protein kinase?
|
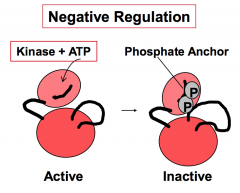
Negative regulation of protein kinase phosphorylation is done by covalent addition of phosphate(s). This phosphorylation promotes an inactive conformation of the kinase (e.g. addition of phosphates to the phosphate anchor.)
|
|
|
Which enzymes reverse the positive and negative effects of reversible protein phosphorylation for regulation of protein kinase activity?
|
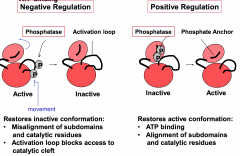
Positive and negative effects of phosphorylation are reversed by the action of phosphatases.
|
|
|
What are the two predominant switch mechanisms for intracellular signal relays?
|
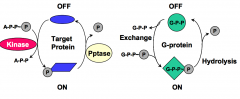
The two predominant switch mechanisms for intracellular signal relays are:
1. Covalent phosphorylation of the target protein by protein kinase 2. Exchange of GDP for GTP by G-proteins |
|
|
What are two key control points in the transition between active and inactive states of G-proteins?
|
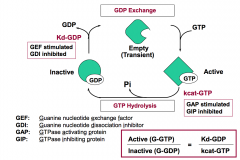
kcat-GTP is the rate of GTP hydrolysis to GDP.
Kd-GDP is the dissociation constant for GDP. |
|
|
Which two regulators stimulate G-protein signaling?
|
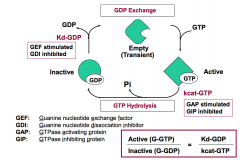
GEFs and GIPs stimulate G-protein signaling by increasing the amount of active G-protein.
GEFs (Guanine nucleotide exchange factors) increase Kd-GDP. GIPs (GTPase inhibiting proteins) decrease kcat-GTP. |
|
|
Which two regulators inhibit G-protein signaling?
|
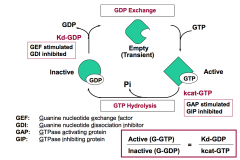
GAPs and GDIs inhibit G-protein signaling by decreasing the amount of active G-protein.
GAPs (GTPase activating proteins) increase kcat-GTP. GDIs (Guanine nucleotide dissociation inhibitors) decrease Kd-GDP. |
|
|
How are polypeptide hormones formed?
|
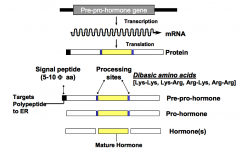
Polypeptide hormones are formed from pre-pro-hormones. Pre-pro-hormones are encoded by genes, transcribed into mRNA, translated into pre-pro-hormones, and processed to give the secreted and active form.
|
|
|
What are the steps by which pre-pro-hormones are targeted to the secretory pathway? Which organelles are involved?
|
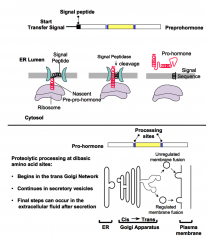
1. An N-terminal signal peptide targets hormone precursors to the rough endoplasmic reticulum.
2. Uptake into the ER lumen occurs during translation. 3. Precursors progress through the Golgi network and are delivered to the plasma membrane in secretory vesicles. 4. Processing to mature forms begins during these transitions. |
|
|
When does a pre-pro-hormone become a pro-hormone?
|
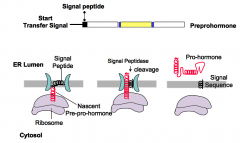
The pre-pro-hormone becomes a pro-hormone upon cleaving of the N-terminal signal peptide. The signal peptide is cleaved upon entry into the lumen of the ER.
|
|
|
How is a pro-hormone converted to a mature hormone?
|
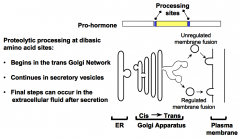
Pairs of basic residues (i.e. Lys-Lys, Lys-Arg, Arg-Lys, or Arg-Arg) mark the cleavage sites. Pro-hormone converting enzymes (PCs) are serine proteases that cleave on the carboxyl side of the dibasic amino acid sites. Processing begins in the trans-Golgi network; may continue in the secretory vesicles. Final steps can occur in the extracellular fluid after secretion has occurred.
|
|
|
What are the strategic features of the pre-pro-hormone mechanism.
|
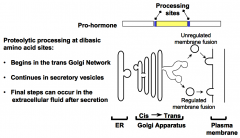
The strategic features of the pre-pro-hormone mechanism are:
1. Ensures transport through the secretory pathway. 2. Delays activation. |
|
|
What are enkephalin precursors?
|
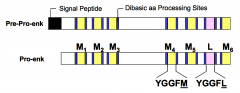
Enkephalin precursors are single genes that encode multiple copies of a single hormone. Enkephalins are pentapeptides with opioid activity, alleviating pain and promoting eurphoric effects. The two types or Met-ENK and Leu-ENK. The enkephalin precursor encodes 6 Met-ENK and 1 Leu-ENK.
|
|
|
What are opiomelanocortin precursors?
|
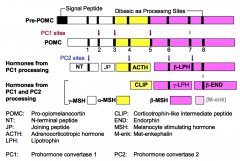
Opiomelanocortin precursors are single genes that encodes several different hormones.
|
|
|
What is Pro-opiomelanocortin (POMC)?
|
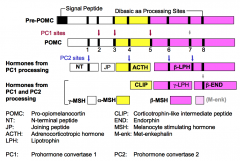
POMC is the precursor of six (*possibly seven) peptides that are hormones with diverse biological activities.
|
|
|
How is POMC processing regulated?
|
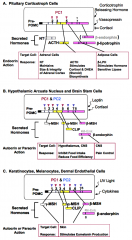
POMC is synthesized in specialized pituitary, brain, and skin cells in humans. Regulation of Pre-POMC production is under control of different hormones in each
cell type. Different mature hormones are secreted by these cell types depending on which prohormone converting enzymes are expressed. |
|
|
What is the endocrine action of insulin?
|
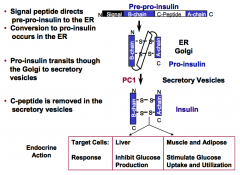
In the liver, insulin inhibits glucose production.
In muscle and adipose tissue, insulin stimulates glucose uptake and utilization. |
|
|
What are the processing steps of the insulin precursor?
|
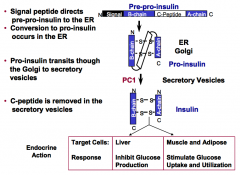
Insulin is produced by the β-cells of the pancreas.
1. A 19 residue N-terminal signal peptide directs pre-pro-insulin to the ER. 2. Cleavage of the signal peptide upon entry of the ER marks conversion to pro-insulin. 3. Pro-insulin transits through the Golgi to secretory vesicles. 4. C-peptide is removed in the secretory vesicles. (C-Peptide is a ~30 a.a. peptide that connects the COOH end of the B chain to the NH2 end of the A chain) |
|

Pre-pro-parathyroid hormone (pre-pro-PTH, below) is synthesized in chief cells of the parathyroid gland in response to low extracellular Ca2+ and/or elevated extracellular phosphate. The precursor is targeted to the secretory pathway so that mature PTH is secreted into the blood stream.
a. What region of the polypeptide transits through the golgi apparatus? b. What region of the polypeptide encompasses the secreted hormone? |

a. Pro-PTH is amino acids 25-115. Nascent pre-pro-PTH is targeted to the secretory pathway by the N-terminal signal peptide. This peptide is cleaved off the polypeptide as it enters the lumen of the ER for transit to the Golgi apparatus.
b. Secreted PTH is amino acids 31-115. Conversion of Pro-PTH to mature PTH occurs in the golgi apparatus by the action of pro- hormone converting enzymes that cleave after pairs of basic amino acids (dibasic amino acid sites). |
|
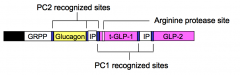
Pre-pro-glucagon is synthesized in the α-cells of the pancreas and in L-cells of the intestinal mucosa. α-cells secrete only glucagon while L-cells secrete only t-GLP-1 and GLP-2.
To account for this cell-type specific pattern of hormone secretion, which of the two indicated pro-hormone converting (PC1 and PC2) enzymes and other protease (Arg protease) must be expressed in α-cells and L-cells? |
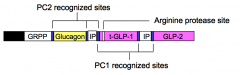
α-cells express PC2; L-cells express PC1 and the arginine protease
|
|
|
An antibody that recognizes the C-peptide region of pro-insulin is available. What species does this antibody detect in plasma?
|
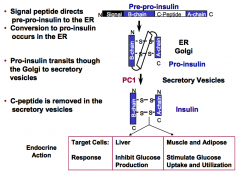
It will recognize two circulating species:
1. C-peptide released from the processing reaction that converts pro-insulin to mature insulin 2. Pro-insulin that might not have been properly processed. (Circulating pro-insulin would be high under circumstances where there is a deficiency in PC1.) |

