![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
87 Cards in this Set
- Front
- Back
|
Sites of Blood Formation
|
2 WEEKS GESTATION: yolk sac is the 1st site of RBC formation
8 WEEKS GESTATION: hematopoiesis begins in spleen, thymus, LN. Major site of RBC formation shifts to liver, increases and peaks at 5 mo, then decreases (liver is the primary organ of hematopoiesis from the third to sixth month of gestation) Liver and spleen continue to produce blood cells into the first week of post-natal life. 4 - 5 MONTHS GESTATION: bone marrow begins |
|
|
Fetus RBC values: RBC ct, MCV, Retic Ct
|
Fetus RBC ct: 1.5 x 10^12 at 12 weeks --> 4.7 at term.
Fetus MCV: mean of 180 at 12 weeks --> to 108 at birth. Fetus Retic Ct: term infants ~5% |
|
|
When do nucleated RBCs disappear after birth?
|
common for nucleated RBCs to circulate freely for several days after birth
|
|
|
Hemoglobin in the Newborn
|
1st few days after birth, Hg levels are much higher in the capillaries than in venous blood due to a loss of plasma from the capillaries. Venous Hg, Hct, RBC ct increases b/w birth and first 3 days of life dt POST-NATAL INCREASE IN PLASMA VOLUME
|
|
|
Erythropoietin (EPO)
|
regulates production of RBCs; produced by the LIVER in the fetus and in early postnatal life. After birth, produced by KIDNEY; production is regulated by tissue oxygenation
|
|
|
EPO changes at birth
|
At birth, Hg ~ 17 g/dL = relative polycythemia dt LOW ARTERIAL PaO2 in utero --> that stimulates EPO production and increases RBC production. At birth, arterial PaO2 rises acutely, resulting in a decrease in EPO. Nucleated RBCs disappear from the peripheral blood and retic ct falls.
|
|
|
RBC life span during the first 6-8 weeks of life
|
is ~90 days instead of nl 120 days
|
|
|
Physiologic Anemia of Infancy
|
occurs because ERYTHROPOIESIS DECREASES DRAMATICALLY after birth. RBC production decreases by a factor of 2 to 3 in the first few days of life and by a factor of 10 in the week following birth; This decrease is initiated by the increase in tissue oxygen level that occurs at birth and is accompanied by a decrease in erythropoietin production
|
|
|
EPO levels in infants
|
EPO level is lowest at 1 mo and highest at 2 mo --> RBC production is lowest at 2nd week then rises to maximum at ~ 3 months.
--> The net result is NADIR at 6 - 9 WEEKS of age (avg.nadir is 2 mo) at level of 9-11 g/dL. |
|
|
Preterm Infant - Hg levels
|
more dramatic fall in Hg than in term. By 2 months:
BW 1.5kg - 2kg: Hg falls to 9.5 BW 1kg - 1.5kg: 9.0 lower the BW, lower the drop. |
|
|
Anemia of Prematurity - etiology
|
impaired (EPO) production in the setting of anemia and decreased tissue oxygen; Also: blood loss from phlebotomy, reduced red blood cell life span, and iron depletion (dt rapid growth)
|
|
|
Anemia of Prematurity - timing
|
AOP typically occurs at 3 to 12 weeks after birth in infants less than 32 weeks gestation.
|
|
|
Physiologic Anemia of Infancy
|
NADIR at 6 - 9 WEEKS of age (avg.nadir is 2 mo) at level of 9-11 g/dL
|
|
|
EPO in older children and adolescents
|
erythropoiesis keeps up with growth, and the mean Hg increases. Valuse for boys and girls diverge at adolescence dt erythroid stimulating effects of andrgens in males (>er Hg)
|
|
|
MCV in older children and adolescents
|
falls during the first 6-12 months of life, when it reaches its nadir of 77 fL, then rises throughout childhood and adolescence.
|
|
|
Reticulocyte ct in older children and adolescents
|
normally <2% after 4 months of age, and nucleated RBCs are not usually seen in the circuclation after the first week of life
|
|
|
Blood Volume in older children and adolescents
|
fairly constant after 6 months of age and remains 75-77 mL/kg
|
|
|
Hemoglobin - structure
|
made up of iron-containing heme and a protein, globin. Hgb is a tetramer made up 2 pairs of globin chains, each attached to an iron-containing porphyrin ring (heme).
|
|
|
Normal Adult Hemoglobin
|
A2B2
|
|
|
Hemoglobin in the embryo
|
Gower-1 and Gower-2, and Portland (they contain zeta and epsilon chains). By 8-12 weeks gestation, the Gower and Portland Hgb's dissapear, and HbF predominates.
|
|
|
HgF values at different ages
|
contains alpha and gamma chains (A2G2).
HgF IN 6 month FETUS: 90% of HgbT; after 6 months begins to be replaced by adult Hg. HbF AT BIRTH: 70% of HgbT HgF AT 4 MO: <20% of HgbT HgF AT 1 YO: <2% of HgbT |
|
|
HbF with hemoglobinopathies or thalassemia
|
HgF elevated
|
|
|
HbA2 or minor Adult Hgb, elevated in which condition?
|
produced in late gestation and accounts for ~2-3% of HgbT after 1st few months of life. ELEVATED in B-THALASSEMIA TRAIT !!!
|
|
|
O2 dissociation curve - shift after birth
|
tied to the intrinsic function of Hgb. In the fetus and Newborn, the O2 dissociation curve favors oxygen extraction from maternal circulation. This limits the proportional release of O2 to the tissues after birth. O2 dissociation curve shifts to the right after birth allows better release of O2 to the tissues; this is due mainly to the change from HbF to HbA and the effect of 2, 3 DPG.
|
|
|
Normal Erythropoiesis
|
involves maturation of stem cells: proerythroblasts --> erythroblasts of different stages --> reticulocytes (lost nucleus but retain RNA) --> mature cells (in periphery have lost their RNA).
|
|
|
How are reticulocytes recognized on a peripheral blood smear?
|
a special stain; (polychromasia)
|
|
|
Reticuloendothelial System and Heme metabolism
|
the spleen removes old or damaged red cells that are then ingested by macs. The Hgb is catabolized, and the porphyrin ring of heme is opened, forming unconjugated bili. Haptoglobin binds and transports Hgb.
|
|
|
Ferritin
|
Direct measure of Iron Levels!
Iron released from heme or absorbed in the intestine from the diet is transported by ferritin. Thf, FERRITIN reflects IRON STORES !! (**ferritin is also an acute phase reactant) |
|
|
Other indirect measures of iron levels
|
Transferrin saturation and TIBC
|
|
|
Classifications of Defective Erythropoiesis
|
1) Production (Proliferative) defect
2) Maturation Defect 3) Survival Defect |
|
|
Defective Erythropoiesis - Production Defect
|
Decreased EPO (renal disease) and BM failure
|
|
|
Defective Erythropoiesis - Maturation Defect
|
1) Cytoplasmic: all are related to impaired Hgb synthesis
- iron deficiency - protoporphyrin deficiency (sideroblastic anemia) - globin synthesis deficiency (thalassemia) 2) Nuclear: DNA synthesis defects (folate, B12 defic) |
|
|
Defective Erythropoiesis - Survival Defect
|
1) Intrinsic (inherited): membrane cytoskeletal protein (spherocytosis, elliptocytosis), metabolic enzyme deficiency (G6PD), or Hemoglobinopathy (SCD, HbC, HbD, HbE)
2) Extrinsic (acquired): autoimmune hemolysis, malaria, DIC, vascular hemolysis |
|
|
Anemia - Lab work up
|
Initial: CBC with smear, retic ct
|
|
|
Retic Ct and Production or Maturation Defect
|
leads to low retic count !!
|
|
|
Retic Ct and Survival Defect
|
shortened red cell survival stimulates a high count as new RBCs are produced.
|
|
|
Value of the peripheral smear
|
can confirm the red cell indices (hypochromia, microcytosis, macocytosis) and reveal specific RBC shapes
|
|
|
Defective Erythropoiesis - Survival Defect
|
1) Intrinsic (inherited): membrane cytoskeletal protein (spherocytosis, elliptocytosis), metabolic enzyme deficiency (G6PD), or Hemoglobinopathy (SCD, HbC, HbD, HbE)
2) Extrinsic (acquired): autoimmune hemolysis, malaria, DIC, vascular hemolysis |
|
|
Anemia - Lab work up
|
Initial: CBC with smear, retic ct
|
|
|
Retic Ct and Production or Maturation Defect
|
leads to low retic count !!
|
|
|
Retic Ct and Survival Defect
|
shortened red cell survival stimulates a high count as new RBCs are produced.
|
|
|
Value of the peripheral smear
|
can confirm the red cell indices (hypochromia, microcytosis, macocytosis) and reveal specific RBC shapes
|
|
|
Proliferation defect - Mechanisms of Anemia
|

|
|
|
Maturation Defect - Mechanisms of Anemia
|

|
|
|
Survival Defect - Mechanisms of Anemia
|

|
|
|
Schistocytes
|
Microangiopathic hemolytc anemia (seen in TTP, HUS, HELLP, DIC, and occas. vasculitis), severe burns, and valve hemolysis
|
|
|
Spherocytes
|
autoimmune hemolytic anemia, hereditary spherocytosis
|
|
|
Target Cells
|
Mainly alcoholics or other significant liver disease, but also seen in thalassemia, and other hemoglobinopathies (Hgb C)
|
|
|
Sideroblasts
|
found in bone marrow of alcoholics and certain types of myelodysplasia
|
|
|
Teardrop Cells
|
myelofibrosis/myeloid metaplasia; also with thalassemia
|
|
|
Burr Cells (echniocytes) vs. Spur Cells (acanthocytes)
|
Burr cells - renal dz (uremic pts)
Spur cells - liver disease |
|
|
Howell-Jolly Bodies
|
Seen in Splenectomy or functional asplenia. Result of fragmentation of the nucleus (karyorrhexis), causing the formation of small black "pellets" this occurs normally and the spleen efficiently removes them.
|
|
|
Hypersegmented PMNs
|
Megaloblastic anemia (pernicious anemia/B12 deficiency, folate deficiency)
|
|
|
Hypoproliferative Anemia dt:
APLASTIC ANEMIA |
dt defect in the pluripotent stem cell leading to pancytopnia; often autoimmune
|
|
|
Hypoproliferative Anemia dt:
PURE RED CELL APLASIA |
disorder char. by an isolated anemia and the absence of red cell precursors in the bone marrow. There is an association with thymomas in adults
|
|
|
Hypoproliferative Anemia dt:
MARROW INFILTRATIVE DISORDERS |
1) Fibrosis 2) granulomas 3) tumor; cause a myelophthisic appearance in the peripheral blood with teardrop red cells and nucleated red cells
|
|
|
Hypoproliferative Anemia dt:
UREMIA |
associated with anemia dt to decreased EPO production and is usually responsive to recombinant EPO
|
|
|
Hypoproliferative Anemia dt:
ENDOCRINE |
Hypothyroid, Addison's, Hypogonadism, Panhypopit:
profound endo failure dt deficiencies in thyroid hormone, glucocorticoids, testosterone, or growth hormone may lead to anemia |
|
|
Hypoproliferative Anemia dt:
ANEMIA CHRONIC INFLAMMATION |
mc hypoproliferative anemia; decreased EPO levels and impaired iron utilization; etiologies: infxns (endocarditis, Tb, osteo), noninfectious: SLE, RA, vasculitis.
|
|
|
Iron Deficiency Anemia - nonhematological effects
|
behavior and learning disturbances
|
|
|
Hypoproliferative Anemia
IRON DEFICIENCY ANEMIA |
defect is intrinsic to the RBC
|
|
|
iron stores in different ages
|
Adult - 5g of body iron
Newborn - 0.25g Childhood and Adolescence - 4.75g of iron must be absorbed **during times of maximal growth (infancy or adolesence) iron req't may exceed the iron accrual rate. |
|
|
Dietary Iron
|
only ~5% absorbed, most children require 10-15mg of iron to maintain a positive iron balance. During infancy this requires use of iron fortified foods
|
|
|
AAP recs to prevent iron deficiency:
|
1) Breast milk should be used for at least the first 5-6 months of life
2) Supplement with elemental iron 1mg/kg/day for breastfed exclusively beyond 6 months of age 3) Premature infants should be supplemented by 1-2 months of age 4) Infants who are not breastfed should receive iron-supplemented formula in 1st year of life 5) Iron-enriched cereals should be among the first foods introduced |
|
|
AAP and cow's milk?
|
avoid during the 1st year of life to prevent occult GI bleeding (cow's milk contains poorly absorbed iron)
|
|
|
Most common reason for iron-deficiency anemia in different ages:
|
Infants --> excess cow's milk
Neonates and young children (1-3 years old) --> inadequate iron intake (dietary deficiency). In children with a nl diet --> bleeding (GIT) **TEST ALL CHILDREN WITH IRON DEFICIENCY ANEMIA FOR OCCULT GI BLOOD LOSS** |
|
|
MC congenital defect of the GIT that causes chronic blood loss in children...
|
Meckel diverticulum
|
|
|
MC worldwide cause of chronic blood loss in children...
|
hookworm infection (necator americanus or ancylostoma duodenales)
|
|
|
Characteristics of RBCs in iron deficiency anemia
|
--> microcytic,
--> hypochromic (decreased MCHC = Hb/Hct x 100). --> Low retic ct, --> low serum iron --> low ferritin (Fe stores) --> Serum binding capacity is increased --> transferrin iron saturation is low |
|
|
Table: ACD versus FeDA
|
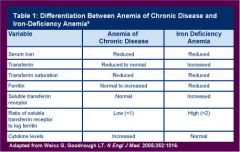
|
|
|
Mentzer Index - why is thal low and FeDA high?
|
RBC ct is generally low in iron deficiency but normal or increased in thalassemia trait.
|
|
|
Other smear features of thalassemia trait
|
basophilic stippling and target cells
|
|
|
Treatment for Iron Deficiency Anemia
|
Iron PO 3-6mg/kg of elemental ferrous iron per day. Most children have no GI upset or constipation
|
|
|
When does reticulocytosis begin with Iron supplementation?
|
3-5 days after beginning therapy and peaks at 7-10 days.
|
|
|
When does hemoglobin increase on iron therapy?
|
will increase 1-2g/dL in the first month
|
|
|
When should you dc iron supplemental therapy?
|
continue even after Hg level normalized in order to insure correction of total body iron deficiet (for at least 1-3 months)
|
|
|
Sideroblastic Anemia - Smear
|
--> RINGED SIDEROBLASTS in the BM = NORMOBLASTS (nucleated RBCs) with iron-laden mitochondria surrounding the nucleus.
--> PAPPENHEIMER BODIES (dark blue cytoplasmic granules of iron) --> INCLUSIONS occur as small single or multiple blue granules (iron) often at the periphery of the cell |
|
|
Sideroblastic Anemia - two populations of cells of peripheral blood smear
|
1) Normal appearing cells
2) Hypochromic microcytic cells. There are 3 kinds: Idiopathic, one subtype of myelodysplastic syndrome, and rare congenital forms |
|
|
Sideroblastic Anemia - Acquired forms
|
longstanding Etoh intox, INH, lead poisoning.
|
|
|
Normal Hg
|
two alpha chains, two beta chains
|
|
|
Thalassemia - definition
|
decreased synthesis or either alpha or beta chains of Hb, unbalanced chain production --> destruction of RBCs and erythroid precursors --> anemia from HEMOLYSIS and INEFFECTIVE ERYTHROPOIESIS
|
|
|
3 categories of B-Thalassemia
|
Mutation in one gene on chromosome 11
1) B-Thal (B): (heterozygotes, Trait) with mild reduction in beta chain synthesis 2) B-Thal (B+) dz:(homozygotes, Thal intermedia): markedly reduced beta chain synthesis 3) B-Thal (B0) dz: (homozygous, Cooley's Anemia, B Thal Major): no detectable B chains |
|
|
B-Thalassemia Minor
|
- Minor or no anemia with disproportionate microcytosis
- asymptomatic except mild anemia common - Hypochromia, microcytosis, target cells, elliptocytes, basophilic stippling - MCV < 75 - RDW is nl **HbA2 >3.5% is diagnostic!!*** |
|
|
B-Thalassemia Major (Cooley's Anemia)
|
Essentially no B-globin production. Remaining highly insoluble alpha-globin precipitates into inclusion bodies (HEINZ BODIES seen with special staining of blood smear). Most erythroblasts die in the BM (intramedullary hemolysis), resulting in erythroid hyperplasia in the BM. Mature RBCs have shortened life span. Nucleated RBCs present.
|
|
|
B-Thal Major - clinical presentation in 6-12 mo
|
by 6-12 mo of age, most infants show pallor, irritability, growth retardation, HSM, jaundice.
|
|
|
B-Thal Major - clinical presentation in children
|
"chipmunk facies"
|
|
|
B-Thal Major - Hgb Electrophoresis
|
shows almost 100% HbF; HbA is absent in homozygous B(0) thal, and is present in very small amts in homozygous and double heterozygous B(+) Thal.
|

