![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
27 Cards in this Set
- Front
- Back
|
Describe the pancreatic islets of Langerhans
|
- 2% of weight of pancreas
- Islands of endocrine cells within the exocrine tissue - Composed of 4 cell types: 1. α-cells = 25% mass and secrete glucagon 2. β-cells = 60% mass and secrete insulin 3. δ-cells = 10% mass and secrete somatostatin 4. PP-cell = 5% mass and secrete pancreatic polypeptide - Formed of a core of β-cells, with a mantle of α-cells and δ-cells |
|
|
Describe intrinsic factors regulating islet of Langerhan secretion
|
- β-cells inhibit their own release (autocrine) and also paracrinely inhibit secretion from α-cells and δ-cells
- δ-cells inhibit themselves alnd also paracrinely inhibit secretions from α-cells and β-cells - α-cells paracrinely stimulate secretion from β-cells and δ-cells (despite its product systemically acting against insulin) - However, blood flows from the centre of the islet outwards and therefore β-cells put their products into the blood before α-cells and δ-cells in the mantle - Consequently insulin largely dictates the regulation of α and δ-cells |
|
|
Describe factors regulating insulin secretion
|
Intrinsic:
- Insulin inhibits itself = autocrine - Glucagon (α-cells) stimulates secretion - Somatostatin (δ-cells) inhibits secretion Extrinsic Stimulants - Glucose in blood = dominant regulator - Amino acids in blood = some can stimulate secretion without glucose, others also require background glucose - Fatty acids or ketone bodies in blood - GI hormones = anticipatory response - Parasympathetic nerves act on muscarinic receptors Extrinsic inhibitors - Sympathetic nerves and adrenaline = act on α2 receptors |
|
|
Describe the factors regulating insulin secretion
|
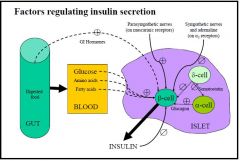
Intrinsic factors
- Insulin itself has an inhibitory action on its own release = autocrine - Glucagon released from α-cells promote insulin secretion - Somatostatin released from δ-cells inhibit insulin secretion Extrinsic promoting factors: - Glucose in the blood is the primary regulating factor - Amino acids in the blood may also promote release of insulin, either alone or with background glucose depending on the amino acid - Fatty acids and ketones in the blood - GI hormones = feed-forward/anticipatory system - Parasympathetic stimulation = on muscarinic receptors Extrinsic inhibitory factors: - Sympathetic nerves and adrenaline (on α2 receptors) |
|
|
How does increased blood glucose cause insulin release?
|
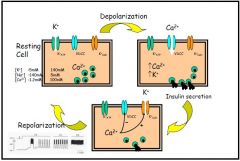
- ATP sensitive K⁺-channels are normally open in resting cell
- Glucose is taken up by the cell and made into glucose-6-phosphate by glucose kinase - Metabolism of glucose-6-phosphate produces ATP within the cell - Increased ATP causes closure of the K⁺:ATP-channel, leading to depolarisation of the cell - Depolarisation promotes the opening of voltage-dependant Ca⁺⁺ channels, causing Ca⁺⁺ to flood the cell - Presence of increased Ca⁺⁺ promotes the exocytosis of insulin granules - The presence of a Ca⁺⁺-activated:K⁺-channel allows the excess K⁺ to leave the cell, causing the repolarisation of the cell, and thus the closure of the VDCC - Decreased ATP in the cell promotes the opening of the ATP sensitive K⁺-channel |
|
|
Describe how sulphonylureas and diazoxide can affect insulin levels
|
- Sulphonylureas and diazoxides act on the K⁺ATP-channel
- Sulphonylureas promote closure of the channel, causing depolarisation and the release of insulin - Diazoxide (also cromakalin and pinacidil) help to keep the K⁺ATP-channel active, causing hyperinsulinaemia |
|
|
Describe how hormones, neurotransmitters and drugs may act, other than on K⁺ATP-channels, to promote insulin release
|
- Protein phosphorylation promotes insulin release via exocytosis
1. Activation of PLC to produces PKC and IP₃ - PKC increases protein phosphorylation, promoting insulin release - IP₃ acts on the ER to release Ca⁺⁺, which binds to calmodulin and promotes protein phosphorylation and insulin release 2. Activation of voltage-dependant calcium channels (VDCC) can also increase Ca⁺⁺ 3. Activation of AC bound to a G-protein causes the modification of ATP to cAMP. cAMP activates cAMP-dependent kinase which promotes protein phosphorylation and consequently insulin release |
|
|
Describe a molecule of insulin and how it is formed
|
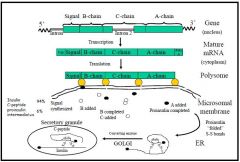
- Insulin is a 6000Da peptide consisting of 2 peptide chains
- 21 AA α-chain is linked by disulphide bonds to the 30 AA β-chain - The α-chain also includes an intra-chain disulphide bond - The gene for insulin is found on chromosome XI - Transcription removes the introns from the gene to form mature mRNA in the cytoplasm, or pre-pro-insulin - Translation forms pro-insulin, a polypeptide with a connecting C peptide between the A and B-chains - The C-chain is a conformational chain which aligns the A and B chains so that disulphide bridges can form between them. It also works as a spacer to make the pro-insulin molecule long enough to span the ribosome-membrane junction - As proinsulin is packaged into its secretory granules, the C-peptide is cleaved to form insulin proper - The amount of C-peptide present is often used as a marker of insulin secretory ability - C-peptides are thought to have a role in kidney function |
|
|
Describe the phases of glucose-stimulated insulin secretion
|
1st Phase:
- Zinc is present in insulin to help it form a hexamer = complexer. This increases insulin's longevity and allows for storage - Within 2 minutes of glucose entering the blood insulin is released - This occurs so fast because insulin vesicles are already preformed and are ready to be secreted 2nd Phase: - The preformed insulin supply is quickly exhaused and there is a dip in secretion - A new, lower peak begins and plateaus as de novo synthesis of insulin begins and more insulin is secreted - In multiple cells these phases are expressed as secretory excursions of insulin following meals - There is a very high peak of insulin secretion after the first meal of the day, but this rapidly falls - The second meal of the day takes longer to reach a less high peak, but longer for levels of insulin to fall - The third meal of the day peaks relatively quickly to about the same level as the second meal, but can maintain the levels of insulin for longer and takes longer to fall as de novo formation is well-established |
|
|
Describe the insulin receptor
|
- Present in most tissues of the body
- Tetrameric glycoprotein = 2 extracellular α-subunits and 2 transmembranous β-subunits - α-subunits contain the insulin binding domain and are bound to the β-subunits and to each other via disulphide bonds, which have a role in insulin binding - The β-subunit is an insulin-stimulated protein kinase capable of autophosphorylation and phosphorylation of other substrates on Tyr residues - Ser phosphorylation of the receptor causes inactivation and is probably PKC mediated |
|
|
Describe the stages in insulin action
|
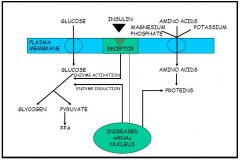
1. Receptor binding
2. Receptor clustering (microaggregation) 3. Early responses - Changes in membrane potential - Protein kinase activation - Generation of second messengers - Phosphorylation / dephosphorylation reactions - Causes: → Enzyme activation = increased glucose made into glycogen and pyruvate. Pyruvate makes more FFAs → Drives uptake of amino acids, K⁺ and magnesium phosphate into the cell, increasing the production of protein → Increased plasma membrane glucose transporters = GLUT-4 moves to the membrane 4. Movement of insulin into coated pits 5. Receptor hormone internalisation / endocytosis = switches off many early responses 6. Delayed responses - Mitogenesis - Gene regulation - Causes: → More proteins made → More enzymes induced, causing the increased production of glycogen and pyruvate |
|
|
In a seriously ill type I diabetic, why should they not be treated with insulin alone
|
- Insulin increases the uptake of K⁺ from the blood
- This dilutes K⁺ in systemic solution - Leads to hypokalemia, leading to ketoacidosis and potentially death |
|
|
What are GLUT proteins? Describe the 4 best-known types
|
- Integral membrane proteins
- Contain 12 membrane spanning helices - Transports glucose (and fructose) across the plasma membrane due to a conformational change - Class I transporters are the best known: - GLUT-1 = basal and non-insulin mediated glucose uptake. Levels in the membranes are increased in low glucose and decreased in high glucose levels - GLUT-2 = expressed by β-cells as prerequisite for glucose-sensing, also small intestine epithelial cells, renal tubular cells and liver cells - GLUT-3 = non-insulin mediated glucose uptake in the brain. Expressed mainly in neurones and the placenta - GLUT-4 = Insulin-stimulated glucose uptake. Found in muscle and adipose tissues for glucose storage. Type II diabetics exhibit a defective recruitment of GLUT-4 in the membrane |
|
|
Describe the fed state
|
- The period 0-4h after a meal
- Anabolic period - Insulin concentration is higher than glucagon concentration - Most tissues use glucose - Increased insulin results in: → activation of glucokinase in the liver to phosphorylate absorbed glucose, which can be used to synthesis liver glycogen and prevent hyperglycaemia → increased glucose uptake by peripheral tissues → increased glycogenesis → increased lipogenesis → increased protein biosynthesis |
|
|
Describe the fasted state
|
- 4-12h after a meal
- Catabolic period - Glucagon concentration is higher than insulin - Brain uses glucose, muscle and liver use fatty acids - Breakdown of liver glycogen stores to provide glucose for the brain = glycogenolysis - Lipolysis = hydrolysis of triacylglycerides from stores to make fatty acids to be used by the muscle and liver - Muscle can also use its own stores of glycogen |
|
|
Describe the starved state
|
- 12h to 40 days
- Once liver glycogen has been used up other sources are required to make glucose - Gluconeogenesis = formation of new glucose from the breakdown of protein (in the muscle) activated by glucagon and later cortisol - Lipolysis produces glycerol which can be used in gluconeogenesis and fatty acids which are used in ketogenesis to provide ketones for the brain |
|
|
Describe the factors regulating the secretion of glucagon from α-cells
|
Glucagon stimulants:
- Amino acids - Ketones - CCK - Incretins (GIP, GLP-1) - Parasympathic activity via the vagus (ACh) - Sympathetic nerves (noradrenaline) Glucagon Inhibitors - Glucose - Somatostatin |
|
|
Describe the main effects of insulin
|
1. Protein metabolism
- increases the uptake of amino acids by most tissues (especially the liver) - increases the rate of protein synthesis by most tissues - decreases the rate of protein breakdown and amino acid oxidation 2. Fat metabolism - Promotes lipoprotein lipase, so that more lipids are broken down and can therefore enter the cell - Increases the pentose phosphate pathway (pentose shunt) so that more NADPH is produced from glucose for fat synthesis = activates lipogenesis - Makes more α-glycerophospate from glucose which is used in fat synthesis - Inhibits hormone sensitive lipase so that lipolysis and β-oxidation is inhibited and plasma FFAs decrease - Inhibits ketogenesis by inhibiting the enzyme that makes FFA-CoA 3. Carbohydrate metabolism - Increases uptake of glucose by peripheral tissues but not the liver - Increases glycolysis via oxidative phosphorylation to produce fatty acids (via the Krebs cycle) and lactate - Inhibits gluconeogenesis by inhibiting the uptake of alanine and glutamine which produce pyruvate, and inhibiting the various enzymes in the gluconeogenesis pathway (except for pyruvate kinase) - Increases glycogen synthesis in muscle and liver = inhibits protein kinase activity, promotes synthase phosphatase activity, causing activation of glycogen synthase - Regulates glycogenolysis by inhibiting protein kinase so inactive phosphorylase kinase = no glycogen made into glucose-1-P |
|
|
Describe the main effects of glucagon
|
1. Carbohydrate metabolism
- Increases glycogen breakdown in the liver only = glycogenolysis (NA and adrenaline increase breakdown in the muscles) - Decreases glycogen synthesis - Increases glyconeogenesis - Inhibits glycolysis 2. Lipid metabolism - Activates lipolysis and β-oxidation, increasing FFAs in the plasma - Inhibits lipoprotein lipase, so less fats are taken up by the cell - Activates ketone body synthesis - Inhibits lipogenesis 3. Protein synthesis - Increases uptake of amino acids (alanine, glutamine) by the liver for gluconeogensis (Cori cycle) - Decreases protein synthesis - Stimulates protein breakdown and amino acid oxidation |
|
|
Describe the effects of insulin deficiency on metabolism
|
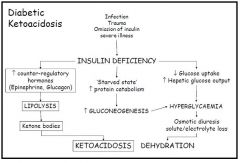
- Decreased ability to use glucose as less glucose absorbed = low tissue availability of glucose
- Glucose remains in the blood → hyperglycaemia - Energy must come from other sources - Lack of insulin means that glucagon and other catabolic hormones are unapposed = predominance of a catabolic process 1. Protein metabolism - Increases breakdown of protein = aminoacidaemia (increased levels in blood) - Increased gluconeogenesis and increased glycogen breakdown - Gluconeogenesis contributes to the hyperglycaemia - Gluconeogenesis also increases urinary nitrogen, resulting in intracellular dehydration (as water follows N), causing polydipsia and potentially coma and death - Intracellular dehydration also causes a loss of K⁺ from the cells and the body, causing hyperpolarisation of the cells that can also result in coma and death 2. Fat metabolism - Decreased lipogenesis in liver and adipose tissue - Mobilisation of fat depot (lipolysis) causing lipaemia (abnormally high level in blood) - Increased ketogenesis in the liver, resulting in ketonaemia - Ketonaemia can result in ketonuria and loss of Na⁺, metabolic acidosis (lacticacidaemia resulting from peripheral circulatory failure can also cause this), and coma and death - Metabolic acidosis can result in hyperpnoea causing extracellular dehydration and further insulin resistances, both of which can lead to coma and death |
|
|
What are the differences between Type I diabetes mellitus and starvation?
|
1. Insulin is absent or very low in diabetes due to disruption of synthesis, but is synthesised and present at low levels in starvation
2. Blood glucose is abnormally high (hyperglycaemia) in diabetes, whereas there is normal blood glucose levels in starvation 3. Diabetes has a large increase in the production of ketone bodies which exceeds the rate of use, resulting in ketoacidosis. Starvation has an increased formatio,but the rate of formation usually equals the rate of use |
|
|
Describe the mechanism of Type I diabetes
|
- Results from a reduction of islet cell mass due to autoimmune disorder
- Need to have a loss of 90% of β-cells before any symptoms of diabetes become present as only need 5% of receptors to be occupied for a full response - Decreased insulin secretion leads to a diagnosis of diabetes - Application of sulphonylureas in this period will give a brief partial recovery of insulin secretion (as some cells still present) but won't stop the autoimmune response - Within a month there is completely absent insulin secretion and death will occur without exogenous insulin - Rapid onset and ketoacidosis |
|
|
Describe the mechanism of Type II diabetes
|
- Develops from insulin resistance due to obesity, a receptor defect, decreased amount of the receptor, or a post-receptor defect
- Causes hyperinsulinaemia and normoglycaemia = 90% develop compensatory increase in β-cell mass and hyperinsulinaemia - Will eventually result in impaired glucose tolerance, clinical symptoms and a diagnosis of diabetes - There may be eventual diminished insulin secretion after many years as the β-cells burn themselves out = results in Type I diabetes - Exercise can increase whole body glucose uptake and metabolism e.g. in exercising there is an increase in the expression of the GLUT-4 transporter and more glucose is therefore transported - 22% of the population in the UK are clinically obese - Also possible to get maturity-onset diabetes of the young = atypical form of Type II diabetes which usually develops before the age of 25. Genetic (autosomal dominant, unlike typical type II) defect causes receptor abnormalities - Usually diet controlled as less severe and ketosis very rare |
|
|
Describe gestational diabetes mellitus
|
- Develops during pregnancy (3%)
- A state of glucose intolerance in the late 2nd trimester - Insulin resistance is increased by large amounts of human placental lactogen (hPL) and growth hormone (GH) - Progesterone and cortisol may also increase resistance - Risk factors include obesity, family history of type II diabetes, and advanced maternal age - Usually resolves post parturition - Type I and Type II diabetes may also occur and these persist - Poor control during pregnancy can lead to: → Macrosomia = abnormally large foetus, can lead to complications → Increased rate of spontaneous abortions → Increased risk of congenital abnormalities. Not a problem in GDM as this doesn't usually occur until weeks 24-26, after organ development → Infant hyperglycaemia during pregnancy can lead to compensatory foetal β-cell hyperplasia. Post-parturition this can lead to neonatal hypoglycaemia |
|
|
What are the signs and symptoms of diabetes?
|
- Elevated blood glucose in the urine (polyuria)
- Dehydration presenting as polydipsia (increased thirst and water consumption) - Inability to use glucose energy can lead to fatigue, nausea and vomiting - More prone to infections of the bladder, skin and vaginal areas - Blurred vision - Extremely elevated glucose leads to lethargy and coma |
|
|
Describe some of the long-term effects linked to diabetes
|
- Blindness = diabetic retinopathy, glaucoma, cataracts
- Kidney failure = diabetic nephrophathy - Nerve damage = diabetic neuropathy - Hardening/narrowing of arteries = atherosclerosis - Stroke - Coronary heart disease - Coma (diabetic ketoacidosis) - Death |
|
|
How is diabetes treated?
|
Non-medical:
- Diet = low cholesterol, simple sugars, calorific intake - Weight reduction and exercise Medication: 1. Increase insulin output = sulphonylureas e.g. tolbutamide, or insulin 2. Decrease glucose production from liver = biguanides e.g. metformin 3. Increase insulin sensitivity = thiazolidinediones e.g. rasiglitazole 4. Decrease carbohydrate absorption e.g. alpha glucosidase Surgical - Transplantation of pancreas |

