![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
31 Cards in this Set
- Front
- Back
|
Describe the Fed state
|
- 0-4hrs after eating
- Anabolic process 1. Increase in plasma glucose causes β-cells in the islets of Langerhans to secrete insulin = insulin to glucagon ratio favours insulin 2. Increased glycogen synthesis in the liver and muscle, increased triacylglycerol synthesis due to increased glycolysis in adipose tissue and fatty acid synthesis in the liver, and increased protein synthesis is stimulated in the muscle (anabolism) 3. Glucose is used by the brain as the sole energy source and uptake is not dependent on insulin 4. Muscle and adipose tissue take up and use glucose but uptake IS insulin dependent |
|
|
Describe the Fasted state
|
- 4-12 hours
- Catabolic process 1. Insulin to glucagon ratio favours glucagon as insulin (and glucose) levels in the blood fall 2. Liver glycogen is broken down to maintain blood glucose promoted by glucagon, mainly to feed the brain. These stores are sufficient to last only 12-24 hours 3. Triglycerides are hydrolysed in adipose releasing FFAs into the blood, which are used preferentially as fuel by muscle and liver 4. Muscles can also use up its own stores of glycogen |
|
|
Describe the Starved state
|
- 12-20 hours onwards
- Catabolic process - Liver glycogen has been used up so alternative substrates are required to provide energy 1. Noradrenaline and cortisol levels increase which activates protein breakdown in muscle releases alanine and glutamine 2. NorA promotes TG breakdown and glycerol release from adipose (rich in sympathetic nerves) 3. Alanine and glycerol etc are gluconeogenic and can be used by the liver to maintain blood glucose which can then be used by the brain 4. The FFAs released from TG are also used by the liver to make ketone bodies which can be used by the brain and muscle for energy |
|
|
How does the liver play a central role in regulating glucose homeostasis?
|
In the fed state:
- Glucose stored as glycogen - Fatty acids synthesised - Plasma lipoproteins synthesised In the fasting state: - Glycogenolysis - Gluconeogenesis - Synthesis of ketone bodies during starvation |
|
|
Describe the main effects of insulin and glucagon
|
Insulin:
- Increase liver glycogen synthesis in muscle and liver and prevents glycogen breakdown - Increase glycolysis - Inhibits gluconeogenesis - Increase glucose uptake in peripheral tissues (not the liver) - Increases amino acid uptake and protein synthesis - Inhibits protein breakdown - Increases lipogenesis - Inhibits lipolysis and β oxidation - Inhibits ketone body synthesis Glucagon - Stimulates glycogen breakdown in the liver (NA and adrenaline stimulate in muscle) and decreases glycogen synthesis - Stimulates gluconeogenesis and inhibits glycolysis - Stimulates protein breakdown and decreases protein synthesis - Increases uptake of amino acids by the liver for gluneogenesis - Inhibits lipogenesis - Activates lipolysis and ketone synthesis |
|
|
Describe the kinetics of glucose uptake into cells
|
- Glucose is too large to diffuse into cells = has 2 transport mechanisms
1. Facilitated diffusion - Mediated by GLUT transporters in the cell membrane - Bind glucose and transport it through the cell membrane via a conformational change in structure - Glucose enters the cell down its concentration gradient 2. Sodium-glucose transporter - Requires energy to transfer glucose against its concentration gradient - Occurs in the epithelial cells of the intestine, renal tubules and the choroid plexus - Movement of glucose is coupled to the concentration gradient of glucose = sodium down its concentration gradient providing the energy required to transport glucose into the cell against its gradient |
|
|
Describe the carbohydrate inputs in glycolysis
|
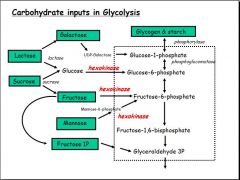
- Lactose must be broken down into galactose and glucose before it can enter glycolysis
- Sucrose must be broken down into glucose and fructose before it can enter glycolysis - Glycogen and starch are the stored source of energy which must be broken down by phosphorylase to glucose-1-P to begin the process of glycolysis - Galactose is also converted to glucose-1-P by UDP-Galactose - Glucose is converted into Glucose-6-P by hexokinase - Fructose and mannose are both converted into fructose-6-P by hexokinase - Fructose-1-P may be converted straight into glyceraldehyde - Phosphorylation (by hexokinase) plays a key role in allowing carbohydrate inputs into the sequence |
|
|
What are hexokinase and glucokinase?
|
- Hexokinase is found in the brain
- Glucokinase is found in the liver - Both enzymes that do essentially the same thing - Both take Glucose + ATP → Glucose-6-P + ADP - This is vital as it allows the glucose to be 'trapped' in the cell (as no transporter for it) and keeps the concentration of free glucose in the cell low compared to the outside, maintaining the concentration gradient = key way of controlling blood glucose - They also phosphorylate other carbohydrate inputs for similar reasons = broad specificity for glucose, fructose and mannose |
|
|
Describe the physiology of hexokinase
|
- Hexokinase is found in the brain and in muscle
- Km for hexokinase is around 0.05mM glucose - Blood [glucose] can be in the region of 5mM which is over 100 times the Km value of hexokinase for the brain - Hexokinase has a high affinity for glucose and therefore blood glucose levels will have to drop significantly before it stops working = ensures that there is always G6P available for energy in the brain - Therefore, hexokinase is usually operating near its maximum velocity, even in low blood [glucose] and is essentially insensitive to fluctuations in [glucose] - In muscle hexokinase is allosterically inhibited by Glucose-6-P = leaves rest of glucose in the blood - Therefore, high G6P signals energy sufficiency and glucose remains in the blood |
|
|
Describe the physiology of glucokinase (liver hexokinase D)
|
- Blood [glucose] around 5mM in fed state
- Hexokinase Km is 20mM - Therefore operates well below its maximum velocity and is highly sensitive to fluctuations in [glucose] - Only begins to generate glucose-6-P when blood [glucose] is high i.e. after a meal - Not inhibited by G-6-P - Therefore glucose is trapped in the hepatocyte and the majority is converted into G-1P and stored as glycogen - As blood glucose returns to resting levels the liver slows its glucose uptake and more glucose stays in the blood to feed the brain and muscles |
|
|
What is glycogen?
|
- Highly branched glucose polymer
- Stored in the cytosol of cells - High concentration in the liver = releaseable into the circulation - High amounts in muscle - used only by the muscles during exercise/fasting etc - Therefore the severity of glycogen storage defects depend on if it effects liver or muscle glycogen - Synthesis and breakdown is regulated by glucagon/insulin through protein phosphorylation cascades |
|
|
What is the main metabolic endpoint of glucose and how does it get there?
|
- ATP for use in the body
- Therefore [ATP] of ATP/ADP ratio can act as signals of energy sufficiency - ATP is produced through oxidation of glucose (CHO) to CO₂ and H₂O - Utilises the central pathway of glycolysis and the citric acid/TCA cycle |
|
|
Describe the key points in the glycolysis pathway
|
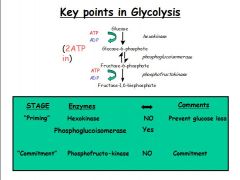
Priming stage:
- Hexokinase = non-reversible step to prevent glucose loss - Phosphoglucoisomerase = reversible step (important in red blood cells) Commitment stage: - Phosphofructokinase = non-revserible step to form fructose-1,6-BP. Once made are committed to glycolysis and there are no intermediates all the way down to glycolysis - Key regulatory step! |
|
|
Describe the role of phosphofructokinase in glycolysis
|
- Makes Fructose-6-P into Fructose-1,6-bisphosphate
- Stages before this in glycolysis are reversible (except for hexokinase) - This stage represents commitment to splitting glucose to pyruvate - PFK is the key regulatory enzyme in glycolysis - Activated by AMP = the hunger signal as indicates that the ATP:ADP ratio is changing and that ATP must be synthesised from ADP - Allosterically inhibited by ATP and citrate = end-products indicating plenty |
|
|
Describe fructose metabolism in the liver
|
- Fructose → (fructokinase) → Fructose-1-P → (phosphofructoaldolase) → Dihydroxyacetone-P and glyceraldehyde
- Products then enter glycolysis or gluconeogenesis - Most dietary fructose is metabolised by the liver, so that there is little left for metabolism in the muscle (which uses hexokinase to convert fructose to F-6-P which enters glycolysis) - The products (triose phosphates) can enter glycolysis below the 2 major control points = tends to overburden the liver with carbon skeletons that are used anabolically to synthesise glucose and triglyceride fats - However, because unregulated and not insulin dependent, excessive fructose ingestion can result in depletion of liver stores of phosphate, limiting ATP production and potentially leading to lactic acidosis |
|
|
Describe the glycolysis 'pay-off' phase
|
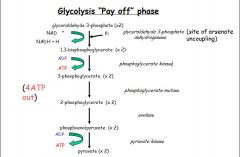
- Energy generating phase
- Two molecules of glyceraldehyde-3-phosphate are converted into 2 molecules of pyruvate with the generation of 4 ATP (substrate level phosphorylation) and 2NADH (which is used in anaerobic glycolysis to make lactate) |
|
|
Describe the energy investment phase of glycolysis
|
- 1st few stages of glycolysis
- Glucose is phosphorylated and cleaved to form 2 molecules of glyceraldehyde-3-phosphate - This process uses 2 moles of ATP to activate and increase the energy levels of the intermediates |
|
|
Describe the regulation of the fate of pyruvate
|
- Fate of pyruvate depends on the presence of O₂
- Anaerobic glycolysis: → Pyruvate is made into lactate via NADH + H⁺ becoming NAD⁺ → No net production of NADH because it is used by lactate dehydrogenase to reduce pyruvate to lactate → Overall only produces a small amount of ATP but valuable energy for cell when oxygen supply is depleted → In cells with no mitochondria (RBCs) NAD⁺ required to perpetuate glycolysis → Lactate can be used to make glucose in the liver when there is lactate acidosis (e.g. during exercise) - Aerobic glycolysis → Pyryvate + CoA → AcCoA + CO₂ → Uses NAD⁺ into NADH + H⁺ and pyruvate dehydrogenase complex → 2 moles of NADH are generated from the oxidation of 1 mole of glucose → Each NADH is oxidised by the electron transport chain to yield 2.5 ATP → Net effect of aerobic glycolysis is generation of 7ATP per mole of glucose (2 directly from substrate-level phosphorylation, and 5 indirectly by oxidative phosphorylation) |
|
|
Describe the Cori cycle
|
- In muscle during intense activity there is only time for anaerobic glycolysis, resulting in a build-up of lactate
- Lactate diffuses out of muscle, and is taken to the liver where it is oxidised to pyruvate, which can then by converted back to glucose via gluconeogenesis - The glucose formed diffuses out of the liver and can return to the muscle to be further used as fuel - This series of reactions, which 'shifts the metabolic burden from the muscle to the liver' is known as the Cori cycle = prevents lactate acidosis |
|
|
Describe the Cori cycle
|
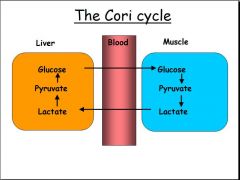
- In muscle during intense activity there is only time for anaerobic glycolysis, resulting in a build-up of lactate
- Lactate diffuses out of muscle, and is taken to the liver where it is oxidised to pyruvate, which can then by converted back to glucose via gluconeogenesis - The glucose formed diffuses out of the liver and can return to the muscle to be further used as fuel - This series of reactions, which 'shifts the metabolic burden from the muscle to the liver' is known as the Cori cycle = prevents lactate acidosis |
|
|
What is gluconeogenesis?
|
- The production of glucose from non-carbohydrate sources
- Glucose is produced from 1. Glycerol = released from triacylglycerol hydrolysis 2. Lactate = from anaerobic glycolysis in RBCs and active skeletal muscle 3. Amino acids = from the breakdown on muscle proteins - Not the reverse of glycolysis = some enzymes are reverse by many high energy steps are irreversible - Key reaction is that carboxylation of pyruvate: Pyruvate + ATP + HCO₃⁻ → Oxaloacetate + ADP + Pi - Pyruvate carboxylase is the enzyme, but biotin required as a co-enzyme - High levels of acetyl CoA stimulates pyruvate carboxylase allosterically = directs back towards storage products as signal of energy sufficiency |
|
|
Describe the citric acid cycle
|
- Otherwise known as the Krebs cycle of tricarboxylic acid (TCA) cycle
- Important for production of energy - Takes place in the matrix of mitochondria - Operates only under aerobic conditions - Final common pathway for oxidation of fuel molecules = amino acids, fatty acids and carbohydrates = all metabolised to acetyl CoA or to other intermediates of the cycle - A cyclical series of 8 reactions that oxidise one molecule of AcCoA completely to 2 molecules of CO₂, generating energy, either directly as ATP or in the form of reducing equivalents (NADH or FADH₂ which are oxidised by the electron transport chain) - Each turn of the cycle produces 10 molecules of ATP so the main pathway for energy generation - Some of the cycle intermediates also exert regulatory effects on other pathways - Important supply of intermediates for biosynthesis |
|
|
Describe the role of pyruvate dehydrogenase in the regulation of the citric acid cycle
|
- Pyruvate enters the mitochondria
- Pyruvate dehydrogenase (PDH) is a multienzyme complex in mitochondrial matrix - Consists of 3 distinct enzymes 1. pyruvate decarboxylase (E1) 2. Lipoyl transacetylase (E2) 3. Dihydrolopoyl dehydrogenase (E3) - Requires 5 co-enzymes: 1. Thiamine pyrophosphate (TPP) 2. Flavin adenin dinucleotise (FAD) 3. Nicotinamide dinucleotide (NAD) 4. Coenzyme A (CoA) 5. Lipoate - Also requires 4 different vitamins as vital components 1. Thiamine for TPP 2. Riboflavin for FAD 3. Niacin for NAD 4. Pantothenate for CoA - Catalyses the irreversible oxidative decarboxylation of pyruvate to produce acetyl CoA and CO₂ - Determines whether or not pyruvate enters the TCA cycle and this 'guards the door' to the cycle - Allosterically inhibited by products = ATP, NADH and acetyl Co-A - Stimulated by AMP = low energy signal/hunger signal - Regulated by phosphorylation by extracellular signals (hormones etc) |
|
|
Describe oxidative phosphorylation
|
- Involves the oxidation fo 2 nucleotides = NADH and FADH₂ by the electron transport chain
- Couples a chemical reaction between an electron donor (NADH or FADH) with an electron acceptor (O₂) to transfer H⁺ ions across a membrane through a set of mediating biochemical reactions - Enzymes that catalyse this reaction simultaneously create a proton gradient across the membrane = high energy state - H⁺ ions are used to produce ATP - O₂ is reduced to H₂O - The electron must be donated to O₂ - if not there is tissue damage as there is no terminal acceptor, so superoxides are produced - Site of certain metabolic poisons e.g. cyanide = competes with O₂ as the terminal electron accetor or acts as an uncoupler in mitochondria |
|
|
Define substrate level phosphorylation and oxidative phosphorylation
|
Substrate level phosphorylation
- Formation of ATP by the direct phosphorylation of ADP - Does not require O₂ and therefore important for ATP generation in tissues short of O₂ e.g. exercising muscles Oxidative phosphorylation - Requires O₂ - The most important mechanism for the synthesis of ATP - Involves the oxidation fo 2 nucleotides = NADH and FADH₂ by the electron transport chain |
|
|
Describe 'Fed' signals
|
- Key intermediates such as ATP, NADH, acetyl CoA and citrate accumulate
- ATP and citrate inhibit PFK allosterically causing G-6P to accumulate - G-6P can be switched into the production of glycogen and pentoses (PPP) which also produces NADPH - Acetyl units can be drawn from the central pathway to synthesise fatty acids |
|
|
Describe the process of fatty acid synthesis
|
- Fatty acid synthesis (lipogenesis) consists of cyclical series of reactions in which a molecules of fatty acid is built up by the sequential addition of 2 carbon units, derived from acetyl CoA, into a growing fatty acid chain
- Requires Acetyl CoA and NADPH - Requires the transport of acetyl units across the mitochondrial membrane (acetyl shuttle/citrate = NOT transporters) since this occurs in the cytosol of the cell - Acetyl Co-A carboxylase is activated by citrate and insulin (signs of plenty) - Requires biotin and NADPH (PPP) |
|
|
Describe 'Fasting' metabolic signals
|
- ATP and NADH low
- ADP and NAD are high - Adrenaline and noradrenaline |
|
|
Describe fat metabolism
|
- Input of fats into the central pathway
- During fasting triglycerides are fed into the central pathway - Catalysed by a hormone sensitive lipase activated by a cAMP responsive kinase and then transported - Stimulated when ATP and NADH are low and ADP and NAD are high - Stimulated by adrenaline and inhibited by insulin - Fatty acid breakdown occurs in 4 stages 1. Lipolysis = hydrolysis of triacylglycerol - Produces glycerol and free fatty acids - Glycerol can be converted into pyruvate or glucose in the liver - Free fatty acids travel in the blood bound to albumin and are taken up by the muscle or liver cells for oxidation 2. Activation of fatty acids - Activated by attachment of acetyl CoA 3. Transport into mitochondria - Via the carnitine shuttle 4. β Oxidation - fatty acids are degraded by a cyclical sequence of 4 reactions which add CoAs, FAD, and NAD - Results in the shortening of fatty acid chain by 2 Cs per sequence - The 2 Cs removed as acetyl CoA - Yields net 131 ATP as every AcCoA produced goes into the citric acid cycle |
|
|
Describe amino acid metabolism by the central pathway
|
- Most proteins in the body are constantly being synthesised from amino acids and degraded back to them
- Starvation may lead to proteolysis - Occurs in the mitochondrial matrix - Activated by ADP, GDP - Inhibited by ATP, NADH and GTP - Key issue for amino acid metabolism by the central pathway is deamination whereby protein nitrogen must be safely disposed - Degraded proteins lose their amino group but ammonia is extremely toxic - Ammonia required to be converted to non-toxic urea by the urea cycle - The urea cycle occurs exclusively in the liver - Fumarate links the urea cycle with the citric acid cycle |
|
|
Describe 3 hormones that help regulate metabolism
|
1. Growth hormone
- Increases protein synthesis - Increases fatty acid mobilisation - Decreases glucose utilisation - Net result increases body protein - Human growth hormone deficiency causes retardation in children - Can be alleviated by giving growth hormone injections 2. Thyroid hormones - Stimulate uptake of glucose, glycolysis and gluconeogenesis - Increases lipid mobilisation from fats and increases oxidation - Lowers cholestrol (more excreted) - Hyperthyrodism causes people to be thin = excitable, don't like heat, can't sleep etc - Hypothyroidism causes sleepiness (somnelsence), increased body weight 3. Cortisol - Increased protein breakdown and reduces protein synthesis - Increases fat breakdown and decreases glucose uptake in fat cells - Excess cortisol can cause obesity (paradoxically) - perhaps by altering appetite? |

