![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
48 Cards in this Set
- Front
- Back
|
Describe the 3 hormones that the thyroid glands secrete and their main regulatory action
|
Basal metabolic rate:
- Thyroxine (T4) - Tri-iodothyronine (T3) - Both based on tyrosine Calcium homeostasis - Calcitonin |
|
|
Describe the microstructure of the thyroid gland
|
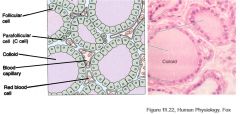
- Made of follicles
- Follicular cells secrete T3 and T4 into the follicle lumen - Parafollicular cells (C cells) found outside follicles and secrete calcitonin |
|
|
What are the 6 steps in thyroid hormone synthesis?
|
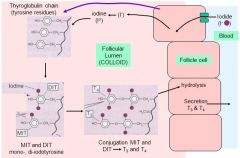
1. Thyroglobulin synthesis
2. Uptake and concentration of iodide (I⁻) 3. Oxidation of I⁻ to iodine 4. Iodination of thyroglobulin 5. Coupling of 2 iodinated tyrosince molecules to form T3 or T4 6. Secretion |
|
|
Why does the thyroid gland trap iodide and how does it do this?
|
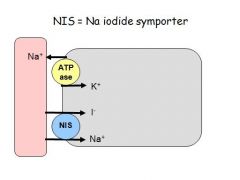
- Iodine is rare
- Follicles actively accumulate iodine (iodide) from blood and secretes it into the colloid - Ingested iodine tends to go towards the thyroid for concentration and use = used for imaging and treatment - Iodide enters the cell by a Na/iodide cotransporter located on the basolateral side of the follicle cell - This is against the concentration gradient = 30x the circulating levels - Iodine can be stored in the thyroid for a couple of months - Iodide is trapped from sea food (sea water), sea salt, fruit and vegetables (depends on soil and imports), and supplemented foods (salt, chocolate) - The amount of iodine in foodstuffs depends on the height above sea level (high = less iodine) and some foods chelate iodine e.g. sauerkraut |
|
|
Describe how thyroid hormone is secreted and circulated
|
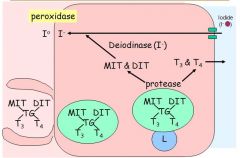
Secretion:
- MIT, DIT, T3 and T4 are bound to a thyroglobulin backbone aftering being synthesised in the follicular lumen - The throglobulin is endocytosed back into the follicular cells - Inside the follicular cells proteases cleave the products from the thyroglobulin. - MIT and DIT are recycled and resecreted into the lumen - T3 and T4 are transported out of the cell and into the blood Circulation: - Thyroid hormones are associated with proteins = most bound to TBG (thyroxine binding globulin) - TBG has a higher affinity for T4 - Only the free T3/T4 can enter the cell - Free hormones are physiologically active - Therefore, although there is much more T4 circulating, proportionally less is free and available for the cells to use, and it must be converted to T3 to bind thr T3R and have an effect (deiodinases) |
|
|
Describe the function and types of thyroid hormone deiodinases
|
- Convert T4 into T3
- Peripheral tissues regulate T3 levels by increasing or decreasing deiodinases - Type 1 = cell surface of most cells → increase local T3 → inner and outer ring deiodination → activated T4 to T3 - Type 2 → Intracellular raises in T3 in CNS and pituitary → outer ring deiodination → activates T4 to T3 - Type 3 → removes iodine from T4 to make reverse T3 (rT3) → Found especially in the plancenta (protect foetus?) and CNS → Inner ring deiodination → Inactivates T4 to rT3, T3 to T2 |
|
|
Describe the actions of T3 on target cells
|
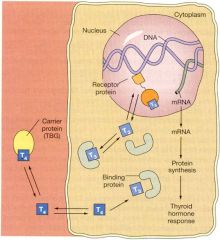
- T3/T4 have to enter the cell via MCT7 (10)
- T4 must be converted to T3 = deiodinases found in most cells, espeically kidney, liver and muscle - Receptors T3Rα and T3Rβ have multiple isoforms and bind to multiple different partners = various effects Physiological actions - Maturation and differentiaton = bones/lungs/brain etc - Neurological functions = synapse formation, myelinogensis, neuronal outgrowth - Growth = regulated by GH but T3/T4 needed to work - Metabolism = effects all metabolism pathways, both anabolic and catabolic. Effects the BMR |
|
|
What are the signs and symptoms of hyperthyroidism and how would you diagnose this?
|
- T3/T4 high enough to cause symptoms called thyrotoxicosis
- Graves disease = auto-antibody TSI binds to the TSH receptor and acts like TSH - Signs/symptoms: → heat intolerance, sweating → weight and muscle loss → increased appetite → diarrohoea → nervous irritability → goitre Diagnosis - High free T3 and free T4 - High TSH suggests fault in or above the pituitary gland - Low TSH suggests a thyroid gland problem (tumour/graves) |
|
|
What are the signs and symptoms of hypothyroidism and how would you diagnose this?
|
- When T3/T4 low enough to cause symptoms called myxoedema
- Hasimotos thyroidtis (auto-immune destruction) - Iodine deficiency Signs/symptoms: → slow/lethargic → overweight/obese → alopecia → goitre Diagnosis - Low T3/T4 (free T3) - Usually high TSH |
|
|
How does T3 alter metabolism?
|
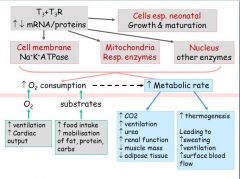
- T3 increases the amount of ATP synthesised
- 40% of basal energy in the cell goes into making ATP requiring increased O2 - To match the increase in O2 requirements T3 needs to: → increase ventilation and respiration → needs to deliver the O2 • increase heart rate • increase blood flow • increase myocardial activity therefore increasing cardiac output - O2 use needs substrates for oxidation = proteins, lipids, carbohydrates for metabolism |
|
|
How does TSH increase thyroid hormone synthesis and secretion?
|
- TSH released from the anterior pituitary
- Regulates thyroid hormone synthesis in all places and at all steps in the cycle - Small TSH increase can cause high T3/T4 outputs |
|
|
How do both low and high T4 induce a goitre?
|
- Iodine deficiency = high TSH and low T4
→ low T4 stimulates high TSH, which normally acts on the thyroid to produce more T4 → thyroid tries to make hormones, but cannot due to ack of iodine → no negative feedback and continual hyperplastic gland activity - Graves disease = low TSH and high T4 → high T4 switches off TSH → production of TSI which acts like TSH and therefore continues to stimulate gland → increased release of T4 and gland hyperplasia → |
|
|
Describe the treatments for a goitre
|
1. Drugs = inhibit production replace hormone
2. Radioactive iodine = destroys the gland 3. Surgery to remove gland - general surgery issues (fitness etc) - vocal cord nerve damage - bleeding - parathyroid gland damage |
|
|
List the phases and timings of the cell cycle
|
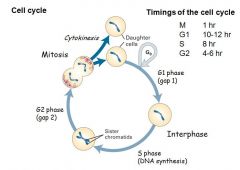
|
|
|
Give 4 factors that control entry into mitosis during the cell cycle
|
1. Mass factor = accumulation of a specific cell mass
2. Growth rate factor = specific growth rates 3. Time factor = timer of oscillator genes 4. Replication factor - completion of S-phase |
|
|
What are cell cycle checkpoints, and what are the 2 most important ones?
|
- Cell cycle checkpoints = molecular processes that monitor progress around the cycle
- G1 checkpoint = restriction point → is environment favourable (growth right etc.) → triggers entry into S-phase? → after this committed to division - G2 checkpoint → is all the DNA replicated → triggers entry into mitosis |
|
|
Describe the molecules which control entry into M-phase (G2 checkpoint)
|
- MPF = maturation promoting factor
- MPF composed of Cdc2 (cell division cycle 2) and cyclin B - Cdc2 is a protein kinase. Levels remain constant in the cell at all time - Cyclin B accumulates and disappears from the cell during the cell cycle - Cdc2 kinase activity requires the binding of cyclin B and therefore is a cyclin-dependent kinase (CDK) |
|
|
Describe the 4 mechanisms that can regulate CDKs and their actions during the cell cycle
|
Regulation of CDKs
1. Association with cyclins 2. Inhibitory phosphorylation 3. Activating phosphorylation 4. Association with CDK inhibitors (CKIs) = hold in inactive state Cell cycle regulation: - Passage through checkpoints is controlled by vaious CDKs - Different CDKs phosphorylate different target proteins - G1-phase CDKs → G1 checkpoint into S-phase = commits to cell division → G1 cyclin and G1 CDK complex → Promotes production of proteins allowing entry into S-phase = Rb (retinoblastoma protein) and E2F - S-phase CDKs → Initially helps in check by inhibitors → Inhibitors degraded by G1 cyclin and G1 CDK → S phase CDKs activated → Triggers formation of replication complexes - Mitotic CDKs → Initally inactive → activation occurs by de-phosphorylation by protein cdc25 → Mitotic CDKs control • chromosome condensation • assembly of mitotic spindle • breakdown of the nuclear envelope |
|
|
Give 3 additional checkpoints other than G1 and G2
|
- Spindle-assembly checkpoint = confirms that all the chromosomes are properly attached to the spindles
- Chromosome aggregation checkpoint = confirms that the chromosomes have segregated to the poles triggering division - DNA damage checkpoint = prevents progression through the cell cycle until the damage is repaired = arrests at G1, S and G2 |
|
|
How do growth factors act on cell division?
|
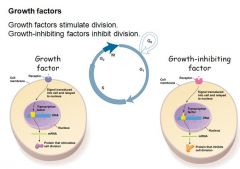
- Growth factors stimulate division
- Growth-inhibiting factors inhibit division - Binding of growth factors stimulates the Ras cascade that leads to Ras-GTP activation and subsequent phosphorylation of transcription factors by MAPK - Binding of inhibitory growth factors(e.g. TGF-β) induces the SMAD cascade where the SMAD-coSMAD complex activates transciption of CKIs, inhibiting division process |
|
|
How can mutations in DNA lead to cancer, and how do mutations arise?
|
- Mutations in DNA lead to changes in protein content/activity
- Mutations can arise in several ways • copying errors during replication • spontaneous cleavage of DNA molecules (purine-backbone bond is very weak) • reaction with chemical by-products • Viruses through the introduction of viral DNA (HIV, Hep B, HPV) • Reaction with genotoxic chemicals • Radiation (UV or ionising) • Repeated trauma (Crohn;s, cirrohosis, OGD reflux, gastritis) - Most cancers require multiple mutations |
|
|
Describe DNA damage checkpoints and repair
|
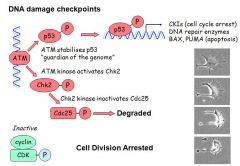
1. ATM kinases move up and down DNA checking for errors and damage
- If no damage is detected then Cdc25 phosphatase activates CDKs causing cell division 2. If damage is detected ATM kinase activates Chk2 3.Chk2 kinase inactivates Cdc25, leading to its degredation 4. This causes the arrest of cell division 5. ATM kinase stabilises p53 = guardian of the genome 6. Stabilisation of p53 causes it to enter the nucleus 7. p53 stimulates CKIs (cell cycle arrest), DNA repair enzymes, and if the damage is too extensive then BAX and PUMA (apoptosis) |
|
|
How can smoking cause cancer?
|
- Cigarette smoke contains benzopyrene
- This undergoes metabolic conversion in the liver to a potent mutagen - Mutagen converts G residues to T residues at DNA hotspots in p53 = p53 loses ability to bind to DNA - Cells lose ability to repair themselves |
|
|
How can DNA mutations lead to cancer?
|
- DNA mutations change protein content/activity which can lead to cancer
- Some cancers form because a protein has a different activity = gain-of-function mutation = oncogenes e.g. ras - Some cancers form because a protein loses its activity = loss-of-function mutation e.g. p53 loss of DNA binding activity = Tumour-supressor genes |
|
|
What is an oncoprotein and how can they cause cancer?
|
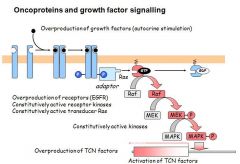
- An oncogene is a gene whose presence can trigger cancer
- Most human oncogenes are mutant forms of normal proteins - The 'normal' gene is called proto-oncogene (prefixed with a 'c') - Most human oncoproteins cause defects in growth factor signalling - Some are hyperactive version of the normal protein - Others have normal activity but abnormally high expression - Oncogenes are dominant mutations = only requires 1 to prevent normal function - Oncogenes arise all the was down the growth factor signalling cascade |
|
|
What are tumour-supressor genes and how can they cause cancer?
|
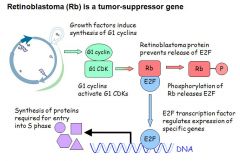
- About 15 tumour supressor genes have been identified
- The normal proteins regulate cell division and promote DNA repair: • provide checkpoints during cell division (Rb, p53, p16) • negatively regulates signalling by growth factors (Wnt-APC) • positively regulates signalling by inhibitory growth factors (SMAD) • contributes to DNA repair (BRCA1) • induces apoptosis (p53, BAX) - Defects in tumour-supressor genes lead to: • unreglated cell division • reduced ability to repair DNA • failure to induce apoptosis - Recessive mutation = requires a mutation in both genes before it loses its function - Retinoblastoma is a tumour-supressor gene. Rb holds E2F (elongation factor 2) in check. E2F regulates the transcription of specific genes required for entry into S-phase - If Rb not produced (genetic) then E2F continually binds to DNA and cell continually enters cell cycle = can't sit in G0 - Thus those with a Rb mutation have a high incidence of cancer at a young age |
|
|
Why does cancer usually require years to develop?
|
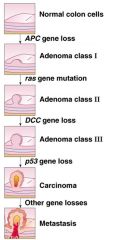
- Cancer is generally a step-wise process (over many years)
- Requires accumulated damage to multiple genes = activation of oncogenes = inactivation of tumour supressor genes - Many cancers have >5 independent mutations - Accumulation of mutations is well-documented for some cancers e.g. colorectal cancer - Development of colorectal cancer: • Inactivation of tumour-supressor genes → Wnt-APC (inhibitor of growth factor signalling) → DCC (inhibitory growth factor signalling) → p53 (cell cycle arrest and apoptosis) • Activation of oncogenes → Ras (activator of growth factor signalling) |
|
|
Describe the mutation rate in cancer cells
|
- The mutation rate in cancer cells is higher than normal
- Several features can contribute to the increased rate: • defects in cell cycle response to DNA damage • failure to trigger apoptosis in response to DNA damage • disruption of mitotic control (genetic instability) • defects in DNA repair enzymes - This mutability has consequences for cancer treatments: • each cancer has a unique set of random mutations • cancer cells can mutate to become resistant to treatments = difficult to produce drugs - However, traditional drugs target DNA synthesis and cell division (to prevent them dividing further) • anti-metabolites • free radicals (damage DNA) • alkylating agents (modify, cleave and cross-link DNA) • topoisomerase inhibitors (prevent DNA from unwinding) • Microtubule inhibitors (disrupt mitotic spindle) - But normal cells synthesise DNA and divide = serious side-effects |
|
|
Describe the morphological differences between the 3 different types of muscle
|
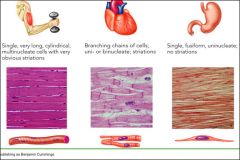
Skeletal muscle:
- Highly organised - Banded/striated - Overlapping strands - Very long cells - Multinucleated Cardiac Muscle: - Striated - Shorter cells which meet at intercalated discs - Branching cells - Usually single nucleus Smooth Muscle: - Disorganised - Single fusiform strands - No striations - Fried egg appearance - Uninucleate |
|
|
Describe the microanatomy of skeletal muscle
|
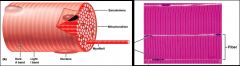
- Sarcoplasm contains glycosomes (granules of glycogen) and oxygen-binding protein called myoglobin
- In addition to normal organelles, cells have myofibrils (actin and myosin), sarcoplasmic reticulum and T-tubules (modifications of the sarcolemma/plasma membrane) - Each muscle fibre is made of many myofibrils that contain contractile elements of the skeletal muscle cells - Myofibrils make up ~80% of the muscle volume - The arrangement of repeating myofibrils creates a series of repeating dark A (anisotropic) bands and light I (isotrophic) bands |
|
|
Describe the sliding filament model of contraction
|

- Thick bands represent myosin filaments
- Thin bands represent actin - The Z band is fixed as is composed of proteins used to anchor myosin and provide something to move against. - Myosin pulls itself along the actin filaments so that I bands are pulled more closely together - Thin fibres at maximum contraction overlap so that a more densely staining centre is produced. - The highest force is generated when partially contracted |
|
|
Describe the features of thick filaments
|
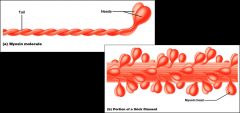
- Thick filaments are composed of the protein myosin
- Each myosin molecule (2 interwoven polypeptide chains) has a rod-like helical tail and 2 globular heads - The globular head is composed of several proteins and works as an ATPase |
|
|
What are actin accessory proteins and what is their role?
|
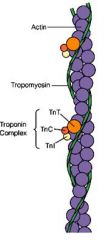
- Actin accessory proteins regulate the interaction between F-actin (filamentous) and myosin
- Composed of 2 sets of molecules = the troponin complex and tropomyosin - The troponin complex composed of 3 proteins: 1. Troponin I TnI = binds actin 2. Troponin T TnT = binds tropomyosin 3. Troponin C TnC = binds calcium - Tropomyosin Tm is composed of 2 fibres that are wrapped into the F-actin complex - Tm regulates acto-myosin interaction - In the absence of calcium Tm prevents myosin (ADP) binding to actin as the troponin complex sits in the binding site - When calcium is present it binds to TnC, causing a conformational changes that causes the Tn/Tm complex to move out of the myosin binding sites on the actin and the myosin heads are then able to bind the actin |
|
|
Describe the cross-bridge cycle for the molecular mechanism of contraction
|
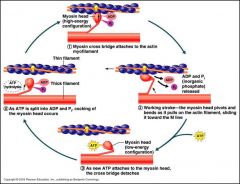
3. The presence of ATP causes myosin to be released from actin
4. ATP is hydrolysed to ADP and Pi by the myosin head (ATPase), which also bind to the head causing a conformational change (cocking) 1. Myosin head free to bind to actin 2. Myosin head pulls itself along the actin filament. ADP and Pi are released from the myosin headgroup so it can perform the 'power stroke'. 3. Loss of ADP means that ATP is now free to bind to myosin, causing it to release actin and the cycle begins again |
|
|
Describe the sarcoplasmic reticulum
|
- An elaborate, smooth endoplasmic reticulum that surrounds each myofibril
- Perpendicular (transverse) channels at the A band - I band junction are the terminal cisternae - T-tubules transmit impulses from the sarcolemma to the SR - The SR regulates intracellular Ca⁺⁺ |
|
|
What are the 3 things that must occur for a skeletal muscle to contract?
|
1. Be stimulated by a nerve ending
2. Propagate the electrical current, or action potential, along its sarcolemma 3. Have a rise in intracellular Ca⁺⁺ levels, the final stimulus for contraction as regulates actin and myosin interaction - The series of events linking the action potential to contraction is called excitation-contraction coupling |
|
|
Describe how a nerve impulse is transmitted at a neuromuscular junction
|
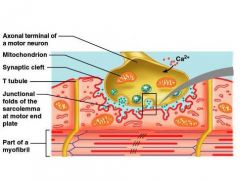
When a nerve impulse reaches the neuromuscular junction:
1. Voltage-regulated calcium channels in the axon membrane open and allow Ca⁺⁺ to enter the axon 2. Ca⁺⁺ inside the axon terminal cause some of the synaptic vesicles to fuse with the axon membrane and release stored ACh into the synaptic cleft (exocytosis) 3. ACh diffuses across the synaptic cleft and attaches to ACh receptors on the sarcolemma 4. Receptors are also channels and binding of ACh on the sarcolemma initiates an action potential in the muscle 5. ACh is quickly destroyed by acetylcholinesterase |
|
|
What is an axonal ending?
|
Synaptic vesicles containing the neurotransmitter ACh
|
|
|
What is the motor end plate of a muscle?
|
A highly folded part of sarcolemma that contains ACh receptors
|
|
|
What is the synaptic cleft?
|
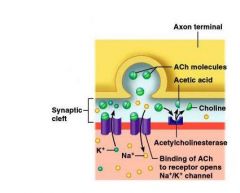
The axonal end and the motor end plate are separated by a space called the synaptic cleft = 1-2nm
|
|
|
Describe the process of excitation-contraction coupling
|
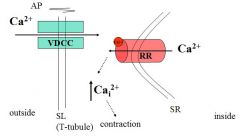
1. The action potential is propagated along (across) the sarcolemma and travels through the T-tubules
2. At the triads (T-tubule closest to the SR) the action potential causes voltage sensitive T-tubule calcium channels to open and release a small amount of Ca⁺⁺ 3. The entry of Ca⁺⁺ activates ryanodine receptors (SR calcium channels) in the SR and causes them to open so that Ca⁺⁺ is released into the sarcoplasm where the myofilaments are |
|
|
Describe the role of Ca⁺⁺ in the contraction mechanism
|
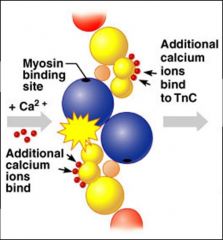
- At low intracellular Ca⁺⁺, tropomyosin blocks the binding sites on actin and myosin cannot attach = this is the relaxed state
- As Ca⁺⁺ levels rise, ions bins to troponin regulatory sites = calcium-activated troponin binds an additional two Ca⁺⁺ at a separate regulatory site (inactive troponin binds 2 Ca⁺⁺) - Calcium-activated troponin undergoes a conformational change. This change moves tropomyosin away from actin's binding sites - Displacement of the tropomyosin allows the myosin head to bind and cycle (attach and detach) - Contraction begins = sliding of thin filaments due to action of the myosin cross bridges |
|
|
Describe the 3 pathways of ATP regeneration
|
As soon as ATP is hydrolysed (4-6 secs) it is regenerated by 3 pathways:
1. CP-ADP reaction Creatine phosphate + ADP → creatine + ATP - Transfer of energy as a phosphate group is moved from CP to ADP – the reaction is catalyzed by the enzyme creatine kinase - Stored ATP and CP provide energy for maximum muscle power for 10-15 seconds 2. Anaerobic glycolysis - When muscle contractile activity reaches 70% of maximum muscles compress blood vessels and O2 delivery is impaired (anaerobic conditions) - Pyruvic acid is converted into lactic acid - Lactic acid diffuses into the bloodstream – can be used as energy source by the liver, kidneys, and heart - Can be converted back into pyruvic acid, glucose, or glycogen by the liver 3. Aerobic glycolysis Glucose + O2 → CO2 + H2O + ATP - Aerobic respiration occurs in mitochondria - requires O2 - A series of reactions where glucose is fully broken down with a high yield of ATP |
|
|
Describe the 3 pathways of ATP regeneration
|
As soon as ATP is hydrolysed (4-6 secs) it is regenerated by 3 pathways:
1. CP-ADP reaction Creatine phosphate + ADP → creatine + ATP - Transfer of energy as a phosphate group is moved from CP to ADP – the reaction is catalyzed by the enzyme creatine kinase - Stored ATP and CP provide energy for maximum muscle power for 10-15 seconds 2. Anaerobic glycolysis - When muscle contractile activity reaches 70% of maximum muscles compress blood vessels and O2 delivery is impaired (anaerobic conditions) - Pyruvic acid is converted into lactic acid - Lactic acid diffuses into the bloodstream – can be used as energy source by the liver, kidneys, and heart - Can be converted back into pyruvic acid, glucose, or glycogen by the liver 3. Aerobic glycolysis Glucose + O2 → CO2 + H2O + ATP - Aerobic respiration occurs in mitochondria - requires O2 - A series of reactions where glucose is fully broken down with a high yield of ATP |
|
|
Describe the 3 pathways of ATP regeneration
|
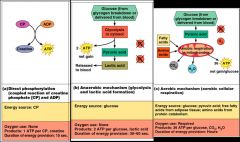
As soon as ATP is hydrolysed (4-6 secs) it is regenerated by 3 pathways:
1. CP-ADP reaction Creatine phosphate + ADP → creatine + ATP - Transfer of energy as a phosphate group is moved from CP to ADP – the reaction is catalyzed by the enzyme creatine kinase - Stored ATP and CP provide energy for maximum muscle power for 10-15 seconds 2. Anaerobic glycolysis - When muscle contractile activity reaches 70% of maximum muscles compress blood vessels and O2 delivery is impaired (anaerobic conditions) - Pyruvic acid is converted into lactic acid - Lactic acid diffuses into the bloodstream – can be used as energy source by the liver, kidneys, and heart - Can be converted back into pyruvic acid, glucose, or glycogen by the liver 3. Aerobic glycolysis Glucose + O2 → CO2 + H2O + ATP - Aerobic respiration occurs in mitochondria - requires O2 - A series of reactions where glucose is fully broken down with a high yield of ATP |
|
|
What is muscle fatigue and why does it occur?
|
- Muscle fatigue – the muscle is physiologically not able to contract
- Occurs when oxygen is limited and ATP production fails to keep pace with ATP use - Lactic acid accumulation and ionic imbalances may also contribute to muscle fatigue - Intense exercise produces rapid muscle fatigue (with rapid recovery). Na+-K+ pumps cannot restore ionic balances quickly enough - Low-intensity exercise produces slow-developing fatigue (with longer recovery period). SR may be damaged, interfering with Ca2+ regulation |
|
|
Give 4 unique physiological features of smooth muscle
|
1. Smooth muscle tone = never completely relexed
2. Slow, prolonged contractile activity 3. Low energy requirements due to 'latch' mechanism 4. Response to stretch e.g. stomach and bladder |
|
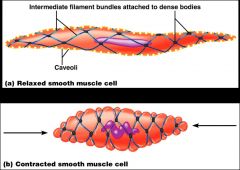
How does smooth muscle contraction occur?
|
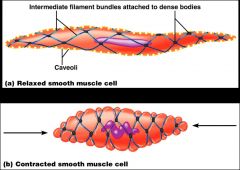
- SR is less developed than in skeletal muscle and lacks a specific pattern AND T tubules are absent
- There are no visible striations and no sarcomeres - Thin and thick filaments are present - Plasma membranes have caveoli - Ca⁺⁺ is sequestered in the extracellular space near the caveoli, allowing rapid influx when channels are opened - Ratio of thick to thin filaments is much lower than in skeletal muscle - Thick filaments have heads along their entire length - There is no troponin complex - Thick and thin filaments are arranged diagonally, causing smooth muscle to contract in a corkscrew manner - Non contractile intermediate filament bundles attach to dense bodies (analogous to Z discs) at regular intervals - Actin and myosin interact according to the sliding filament mechanism - The final trigger for contractions is a rise in intracellular Ca⁺⁺ - Ca⁺⁺ is released from the SR and also moves from the extracellular space into the cell - Ca⁺⁺ interacts with calmodulin and myosin light chain kinase to activate myosin |

