![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
14 Cards in this Set
- Front
- Back
|
Kinetic Energy |
Energy of motion |
|
|
Potential Energy |
Stored Energy |
|
|
Chemical Energy |
Energy stored inside bonds of a chemical compound. Gets released during chemical reaction. |
|
|
Law of Conservation of Energy |
Energy can't be created or destroyed. It only changes form. |
|
|
Temperature |
Measure of Average Kinetic Energy of all of the particles in an object |
|
|
Thermometer |
Instrument used to measure the Average Kinetic Energy of an object |
|
|
Kinetic Theory of Matter |
1) Matter consists of constantly moving particles 2) Faster movement= higher energy |
|
|
Thermal Energy |
Higher temperatures causes particles to move faster and collide which produces energy Ex. Heat from a fire |
|
|
Heat |
Flow of energy from a warm object to a cooler object |
|
|
Conduction |
Transfer of energy through direct contact between objects. (Ex. metal spoon in boiling water) |
|
|
Convection |
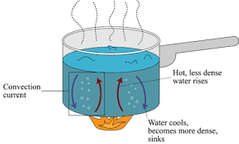
Particles in fluids (liquids/gasses) with a lot of heat move to take the place of particles with less heat. This heat transfer is convection |
|
|
Radiation |
Transfer of energy through air/space in wave form. (Ex. Sun's rays radiate towards earth.) |
|
|
Conductor |
Object that is good at transferring heat. |
|
|
Insulator |
Object that does not transfer heat well. |

