![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
35 Cards in this Set
- Front
- Back

Glycine
|

-Functions directly as a neurotransmitter
-->Acts to block impulses traveling in the spinal cord to stimulate skeletal muscle -Conjugated to cholesterol derivative to form glycocholic acid (bile salt) -->conjugated to cholesterol derivative to form glycocholic acid, which is involved in digestive process. bile salt used to emulsify fat in our diets. -Incorporated directly during de novo synthesis of purines -Condenses with succinyl-CoA in the first step of heme biosynthesis |
|
|
Bile Salt derived from?
|
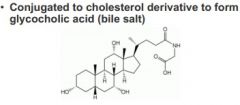
Glycine and cholesterol
(note gylcine stuck on end) conjugated to cholestrol derivative to form glycocholic acid, which is involved in digestive process. bile salt used to emulsify fat in our diets |
|
|
Amino acid involved in porphyrin biosynthesis
|
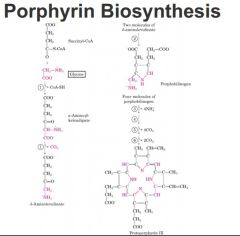
Glycine!
don't memorize, just note that starting with succinyl co and a series of steps end up with protoporpherin 9 glycine derived atoms outlined in red coordinate iron in center and have heme |
|
alanine
|
-Along with glutamine, a predominant circulating amino acid
-Transports amino groups from muscle to liver (transamination from muscle pyruvate) in a process called the glucose-alanine cycle |
|
|
Glucose alanine cycle
|
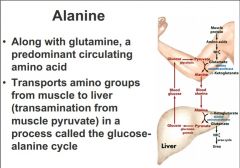
alanine importnat for transport of amino groups from muscle to liver through this glucose alanine shuttle
muscle protein being scavanged have to deal with amonia thats being released combine with pyruvate to get alanine which can travel through the blood to the liver and then be reversed, can deal with this through urea cycle to get urea. then glucose goes back and does the reverse *important: movement of ammonium groups through the body that have arose through transamination |
|
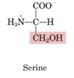
Serine
|
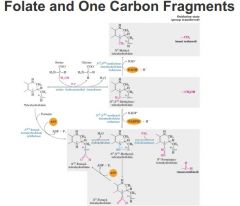
-Source of one-carbon fragments (folate derivatives) used in biosynthesis
-tetrahydrofolate on far left, serine in red.. gives rise to various folate derivatives Goes from most reduced (top) to most oxidized (bottom) introduce single carbon units onto folate derivatives. These will then get transferred in biosynthetic processes that involve single carbon additions other than methylation are from serine side chains from these folate derivatives. |
|
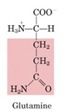
Glutamine
|
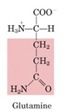
-Along with alanine, a predominant circulating amino acid
-Transporter of amino groups; serves as a form of activated ammonium ions; source of NH4+ in the kidney -Amino group donor in purine biosynthesis -Amino group donor in biosynthesis of amino sugars -Key reaction is Fructose-6-P + glutamine --> glucosamine-6-p + glutamate *All other amino sugars are derived from glucosamine-6-phosphate |
|
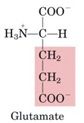
Glutamate
|
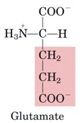
1. Neurotransmitter
-Glutamate is the primary neurotransmitter in the central nervous system Acts on both ion channels and G-coupled receptors 2. Source of GABA (γ-amino butyrate) via decarboxylation of the a-carbonyl. -GABA is the major inhibitory transmitter in the brain -GABA receptor is target of benzodiazepines/barbiturates 3. Participant in transaminations -Glutamate is the source of amino groups for most other amino acids 4. Reactant in ammonia fixation, which results in the production of glutamine |
|
|
What is GABA? What is it made from?
|
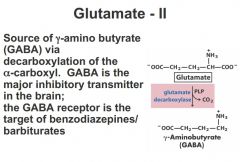
taking glutamic acid, 1 step decarboxylation gives you GABA
PLP is perioxidal phosphate. cofactor. see PLP involved in a lot of these small decarboxylation steps |
|
|
source of amino groups for most other amino acids
what type of reactions allow the transfer of amino groups from this amino acid to form the others? |
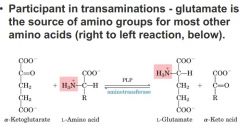
Glutamate
Transamination rxns where we have keto acid and amino group on amino acid. aminotransferases move amino group from amino acid and get a new keto acid. |
|
|
Glutamate dehydrogenase
|
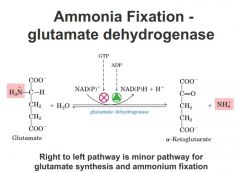
|
|
|
Polyamines
|
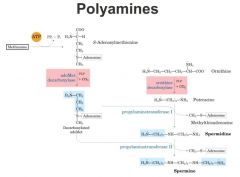
Spermidine
Spermine here is methionine and reacts with ATP, releasing pyrophosphate and a phosphate. We've attached adenosine onto the side chain of methionine giving us s-adenosylmethionine. This is now the donor for these methyl groups as we saw in the donation of methyll groups converting phosphatidyethanolamine to phosphatidylcholine. There is a lot of other methylation reactions, almost all of them the methyl donor is s-adenosylmethionine besides being the methyl donor, s-adenylmethionine can be decarboxylated to give rise to give rise to decarboxylated s-adenosyl methione which itself can give rise to what are called the polyamines (spermidine, spermine, putrescine) these are called the polyamines because they have multiple positively chared amine groups on them important in a number of functions, including compaction and packing of nucleic acids. have very negatively charged phosphodiester backbone and these polyamines can cancel out the charge and bring it together putricine very pungent smelling. rotting meat. |
|
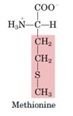
Methionine
|
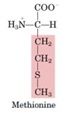
-Activated by reaction with ATP to form S-adenosyl methionine (SAM)- source of methyl groups for most methylation reactions
- SAM can be decarboxylated to leave a propylamine residue attached to the sulfur - precursor of spermine and spermidine (“polyamines”) |
|

Arginine
|

-Immediate precursor of urea production via action of arginase in urea cycle
-Source of NO, a second messenger -->Cofactors include flavin and tetrahydrobiopterin |
|
|
NO synthesis
|
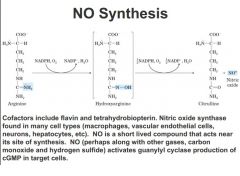
arginine through a two step process makes NO
short lived compound acts near sie of synthesis NO + other gasses activated cGMP involved in vasorelaxation also note that cofactors involve flavin and tetrahydrobiopterin ** important |
|
|
What amino acids contribute to the synthesis of creatine and phosphocreatine?
|
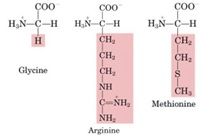
Glycine, arginine, and
methionine all contribute to the synthesis of creatine and phosphocreatine |
|
|
Energy buffering system in skeletal muscle cells?
|
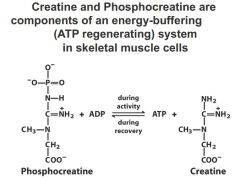
Creatine and Phosphocreatine are
components of an energy-buffering (ATP regenerating) system in skeletal muscle cells in skeletal muscle tissue during rest have build up of phosphocreatine. during burst of energy cellular levels of ATP start to be depleated, cannot be regernated fast enough so you get get ATP via dephosphorylation of phosphocreatine |
|
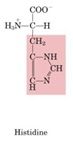
Histidine
|
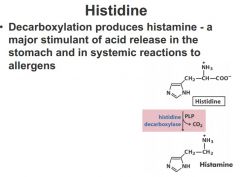
Decarboxylation produces histamine, a major stimulant of acid release into the stomach and in systemic reactions to allergens
Histidine has amidizole side chain what we're looking at is decarboxylation of the alpha-carboxyl group and PLP as cofactor give rise as histamine major stimulant in release of stomach acid as well as gives rise to reactions to allergins take an antihistamine trying to block the action of histamine histamine is an important small molecule. it's a one step derivative away from histidine. |
|
|
What is the relationship between phenylalanine and tyrosine?
|
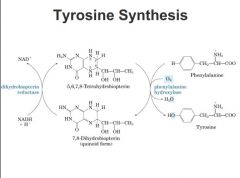
Under normal conditions, phenylalanine is a dietary essential amino acid, but tyrosine is not since it is produced from phenylalanine:
tetrahyrdobiotpertin (same cofactor from NO production from arginine) also a cofactor in conversion of phenylalanine to tyrosine every time convert phenylalanine to tyrosine we convert tetrahydrobioptern to dihydrobiopterin form but this needs to be regenerated for the next cycle and that uses the dihydrobiopterin reductase using NADH as the reducing eqivalent. Tetrahydrobioptern/dihydrobiopterin is cycling make dihydropbioter need to use NADH to convert back cycling every time. |
|
|
Biopterin Cofactor
|
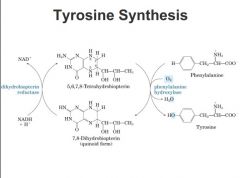
• Provides electrons for reduction of oxygen
in hydroxylation of phenylalanine- regeneration requires specific reductase • Deficiency of phenylalanine hydroxylase (phenylketonuria, PKU) is usually screened for at birth (blood sample to measure elevated phenylalanine levels) - controllable by specialized diet with limited phe, and tyr supplementation - less required by puberty • Deficiency of the biotperin reductase is more severe and cannot be controlled by dietary means -->if you're missing enzyme on left MUCH more severe. ability to recycle and use tetrahydrobiopterin essential not only here but also other dietary processes require it as well, nont just phenylalnine --> tyrosine. |
|
|
Neurotransmitter
|
• Molecule that is synthesized and stored in synaptic vesicles in neural cells
• Release is triggered by an action potential • Bound & recognized by target cell • Activity can be regulated |
|
|
What does bile salt do in our diets?
|
Emulsify fat
|
|
|
Folate
|
Derived from serine!
|
|
|
Transport amino groups
|
-Glutamine
-Alanine |
|
|
Cofactors for nitric oxide synthase
|
flavin and tetrahydrobiopterin
|
|
|
Primary neurotransmitter in the CNS?
|
Glutamate is the primary neurotransmitter in the central nervous system
Acts on both ion channels and G-coupled receptors |
|
|
MSG
|
MSG monosodium glutamate
-Glutamate is an excitatory amino acid -neurotransmitter function can activate ion channels and g coupled receptors -Added to foods as flavor but some people react adversely to it |
|
|
PLP
|
PLP is pyridoxal phosphate. it's a cofactor.
carries out DIFFERENT FXNS involved in decarboxylation fxns, also transamination same cofactor, v different reaction |
|
|
NO is made from
|
arginine
|
|
|
What's more serious - deficiency of phenylalanine hydroxylase or biotperin reductase? Why?
|
• Deficiency of phenylalanine hydroxylase (phenylketonuria, PKU) is usually screened for at birth (blood sample to measure elevated phenylalanine levels) - controllable by specialized diet with limited phe, and tyr supplementation - less required by puberty
• Deficiency of the biotperin reductase is more severe and cannot be controlled by dietary means -->if you're missing enzyme on left MUCH more severe. ability to recycle and use tetrahydrobiopterin essential not only here but also other dietary processes require it as well, nont just phenylalnine --> tyrosine. |
|
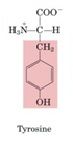
Tyrosine
|
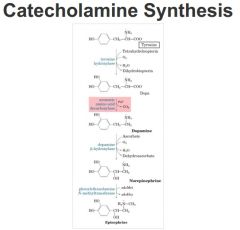
-Made from phenylalanine
-Tyrosine is a precursor of several important molecules: dopa (dihydroxyphenylalanine) --> dopamine --> norepinephrine --> epinephrine -Tyrosine is a precursor of thryoxine (T4)/T3 (active form) -->tri-iiodothyronine (T3) is formed by iodination and condensation of tyrosine residues in thyroglobulin followed by proteolysis of the protein and hormone release -Melanin is derived from tyrosine by oxidation of dopa catalyzed by tyrosinase |
|
|
Dopamine
|
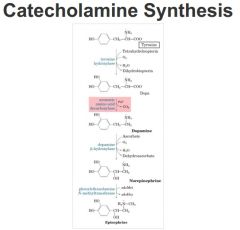
Synthesized from tyrosine
- Intermediate in synthesis of epinephrine/norepinephrine as well as a neurotransmitter - involved in control of voluntary movement (Parkinson’s disease). -Formed by decarboxylation of dopa. -Both epinephrine and norepinephrine are neurotransmitters and signaling molecules (fight or flight response - sympathetic nerves) |
|
|
Thyroid Hormones
active/inactive form? how is it formed? |
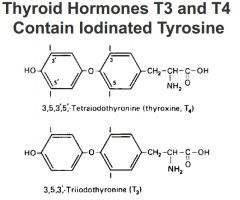
• Active form is tri-iodothyronine (T3; T4 is thyroxine). This is formed by iodination and condensation of tyrosine residues in thyroglobulin followed by proteolysis of the protein and hormone release
-t4 has 4 iodination groups t3 has 3 |
|
|
Tryptophan
|
- Hydroxylation (5-position) followed by decarboxylation yields serotonin, a multifaceted neurotransmitter.
-The hydroxylation step requires Biopterin cofactor; decarboxylation step requires PLP -Some tryptophan can be degraded to nicotinic acid (precursor to NAD+() but this is insufficient to eliminate dietary need |
|
|
Serotonin Biosynthesis
|
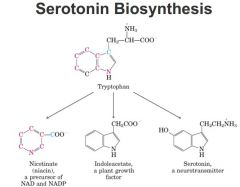
tryptophan double ring structure
to make serotonin can decarboxylate it then hydroxylate it makes serotonin requires PLP and tetrahyrdobiopterin some can be converted to nicotinic acid but at too low of lelves to eliminate niacin from our diets through PLP --> decarboxylation and cofactor can convert tryptophan to serotonin plants can take tryptophan and through deacetylation make indoleacetate which is an important growth hormone involved in the tropisms of plants (down to gravity up to light) to small degree can convert tryptophan to niacin but not enough to eliminate it from our diets |

