![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
219 Cards in this Set
- Front
- Back
|
How do Viruses produce disease?
|
1. effects on cells
2. the way they spread through the body 3. The immune response to the virus 4. The non-immunological factors influencing virus host interactions |
|
|
The effects of Viruses on Cells.
|
There are many kinds of interactions mediated by the genetic makeup of the virus and host cell concerned.
1. Cytocidal effects include a. morphological changes including CPE (cytopathogenetic effect or gross morphology shifts), inclusion bodies, and syncytia. b. Biochemical changes including primary (shut down host protein synthesisin favor of virus) or secondary (reduction and eventual shutdown of host RNA/DNA synthesis) effects. |
|
|
Pathogenic vs. Virulent
|
Pathogenic- a virus can infect tha host and produce signs and synptoms of disease (infect is different than disease as many infections are subclinical)
Virulence- a compareitve measurment of how regularly a virus produces disease in a host in which both strains are pathogenic. Note that both are only valid in-vivo |
|
|
The way in which viruses spread through the body and casue disease
|
1. route of entry- Scratch/injury/skin, conjunctiva, UG tract, GI tract, and Respiratory tract (most common). Note that penetration can be the result of attachment to receptors or direct injection.
2. Mechanisms of spread- local spread on epithelial surfaces, subepithelial invasion and lymphatic spread, spread via blood (main vehicle in systemic disease), CNS invasion. Note that most maternal infections arenot harmful tot he fetus, however some viruses can cross the placenta. Not all are fatal/abortive. |
|
|
Virus Shedding
|
Note that some infectious viruses are not shed in man (like rabies) so they are dead end infections.
Via skin indirect contact Via respiratory tract ie...saliva, mucus, fluid etc...coughing, sneezing, or talking. Via GI tract- Fecal route VIA UG tract- shed in urine Other- Via milk, semen, blood. |
|
|
Host Response- Immunity
|
A. Recovery:
Cell Mediated immunity- The major early response involved CD8+ cells (note that viral antigens are present on cells infected by both enveloped and non-enveloped viruses, and may appear before complete virion synthesis), also brings about T-cell mediated delayed hypersensitivity, Macrophages (although some viruses can infect and live long termin macrophages), and NK cells (induced by IFN it is the first line 1-2 days post infection) Antibody Mediated Immune Cytolosis- later in infection host cells can be lysed bia complement mediated cytotoxicity and cell mediated. Non-antibody dependant complemetn activation- Some viruses directly activated complement. All of the 3 above are the earliest immune responses leading to recovery. For the most part they are directed to the virus infected cells, not the virus itself. B. Immunity to reinfection: Antibody mediated immunity can exert specific anti-viral effects via Classical Neutralization (generally do not interfere with adsorption but with uncoating), Opsonization, and enhancement of Neutralizaton by Rh factor (Ab direted against viral antigen/ab complex) IgG is the most effective defense against viral reinfection as it is the most long lasting. Secretory IgA is short lived (a few years at most). Mostly IgG results form T-cell processing of the antigen. C. Immunopathology: The immune response may contribute to the pathology of infection as a result of Delayed type hypersensitivity (inflamation/mononuclear cell accumulation), Immune complex formation, and immunocytolosys (destruction of infected host cells and thus tissue damage) |
|
|
Non-immunological factors affecting Virus-Host interactions
|
Anatomic barriers as described elsewhere
Fever- replication is strongly influence by temperature Inflamation- Leads to local hyperthermia, and altered local pH. Interferon- ramps up viral immune response. |
|
|
What are the classes of Intereron
|
IFN-A: AKA Leukocyte IFN, TYPE I. Produced by T, B, and O cells are induced by Mixed leukocyte reactions and viruses. All cells have receptors for it. Approved for the Tx of Hairy Cell Leukemia, Kaposi's Sarcoma (AIDS/HHV8), Papilloma virus, HEP C (tx with Peg IFN + ribavirin), as an adjunct to cancer therapy, for Non-hodgkins lymphoma (assoc /w HCV)and may eliminate the carrier state in some HEP B cases.
IFN B- AKA Fibroblast IFN, Type I. Produced by fibroblasts and epithelial cells in responst to the presence of Ds DNA. All cells have receptors. They are approved for the tx of relapsing-remitting Multiple Sclerosis. IFN G- AKA Immune IFN, Type II. Produced by T and NK cells in response to antigens or mitogens. Immune cells have receptors. Approved for the Tx of Chronic Granulomatous disease. |
|
|
Interferon Induction/efects
|
Interferon production is induced by activation of Toll receptor signaling via various antigens including DsDNA
The free unmethylated (Viral) DNA can be directly detected via Toll 9 (as opposed to our own methylated DNA). If infected, the RNA used by viruses lacks a cap and poly A tail, so that as it tries to swipe a cap it activates a nucleic sensoty which activates Interferon response factor 3 (transcriptional transactivator) causing interferon production. The resulting IFN can excite a receptor on the same cell, or naother cell that has not seen a molecular marker of infection (auto or paracrine). The IFN A and B activates STAT 1 and 2. G activates STAT 2 and 3. STAT= Signal Transducers and Activatoros of Transcription. Activation involved phosphorlation and translocation to the nucleus. Translocation activates many genes causing antiviral, antiproliferative, and immunoregulatory responses. The resultant effects are caused by: PKR- Protein Kinase RNA dependant. Inhibits transduction of mRNA into protein by interfering wtih GEF (guanine elongation factor) which is necessary to form the charged metTRNA complex. By phosphorylating EIF2 it drives the reaction towards the uncharged state. 2'5'OAS- 2-5 Oligo A Synthetase. This produces 2'5' Adenine Triphosphate dimers (regular adenine has a 3'-5' linkage) which activates the already present RNAse L, killing any RNA virus, but other RNA as well. Adenine Deaminase- Removes the amino group from adenine in RNA which then becomes inosine (codes for G-C rather than A-U) so it alteras th code in ANY RNA that has an A. MX protein: A GTPase which depletes the cell's GTP (an important energy source for viral transport, esp in influenza). Inducable NO synthetase- Induced primarily by IFN-G, it takes the place of other ROS in chronic granulomatous disease (pt is unable to make H2O2). |
|
|
IFN therapy
|
IFN used in therapy can be:
Endogenous- with polyriboinsinic/Polyricocytidylic acid stabilized with carboxymethylcellulose and Poly L-lysine complex. Exogenous derived naturally and via recombination. Pegalated Interferon is also available- The additon of polyethylene glycol (antifreeze) creates in IM varieyt that lasts far longer than IV or even non-pegalated IM varieties. There are some concerns with toxicity. Note that some viruses become resistant by coding for inderteron decoyes that interfere with the molecules that IGN induces. |
|
|
Epidemiology of Influenza A
|
Influenza A is the major casue of epidemic (B causes localized outbreaks and does not have teh same dramatic mutation pattern as A, C is a very mild ilness and is not as clinically important).
It is the extremely high rate of mutation of Influenza A, and the animal reservoirs that can grow it, which permit the possibility of an epidemic. 25% of all office visits are due to respiratory problems, and 50% of that is due to influenza. |
|
|
Influenza, Clinical Disease characteristics
|
The course is generally (but not always) self limiting, so that it infects millions per year wtih significant morbidity/mortality.
Signs/Symptoms: The signs are more global and constitutive than most respiratory invading viruses; Inluding fever, cough, H/A, myalgia, and anorexia...all of which outweight the resp symptoms. Dx: Via Clinical Signs Near Pt tests including point of care tests that measure viral antigens or enzymes in nasal secretions or saliva, Dx in 15 mins. We can also examine Ab titers. Mechanisms of Illness: Respiratory cilia paralysis, Destruction of Type II pneumocytes (elimination of surfactant production), and the cytokine storm superinflamation can lead to multi organ failure and vulnerability to 2ndary infection. Viremia is rare, but thoe with cystic fibrosis, asthma, and other cardiopulmonary insufficiencies are at higher risk. Thus we vaccinate them first. |
|
|
Influenza/bacterial synergy
|
The Influenza HA receptor needs to be cleaved by an extracellular protease, after which it binds to Sialic acid, exposed and cleaved by the influenza NA.
NA's removal of mucus and exposure of SA also exposes bacterial receptors for adhesion. Further the paralysis of cilia, and loss of surfactant allow the bacterial to persist in the area as well. The infection with bacteria leads to the presence of extracellular bacterial proteases and caspases as well, all of which increase HA clevage and viral infectivity...thus a viscious cycle. |
|
|
Influenza Structure
|
Influenza is a segmented -RNA Virus with an outer envelope. The envelope surface is studded with HA0 (Hemagglutinin) and NA (Neuraminidase).
HA0 needs to be cleaved to HA1 and HA2 joinded via a disulfide bond by an extracellular protese. 3 of those units associate to from a trimer called HA, whcih will bind Sialic Acid. Neuraminidase clears mucus and reveals sialic acid which is also cleaves unless HA binds to it. It is required for penetration, but more so for viral escape after synthesis. The Genome consists of 8 segments of - stranded RNA, each encoding for a viral protein. Also inside the virus we have Viral associated RNA polymerase. |
|
|
Influenza Vaccine
|
Consists of Purified Hemagglutinin derived from both Influenza A and B grown in chick embryo. Thus it is a killed vaccine, and each year it is updated based on which strains are determined to be most prevalent.
Alternative vaccines include subunit extracts of the viral particle, and a lise cold adapted vaccine that can be administered intranasally (mostly for kids). The vaccine is only moderately effective and protection is not absolute. It is recommended for those at high risk ie...Heart disease, chronic bronchopulmonary disease, and those at risk of exposure and to expose others (Health care workers) as well as the aged and young. Note that the threat of a new more infectious mutant indicates immunization for eveyone as soon as it is availalble. Prophylaxis with antivirals may also be effective in preventing the disease. |
|
|
Hemagglutination Inhibition
|
HI- HA agglutinates RBCs, which activity is blocked by nutralizing antibodies as measured by the HI titer. A rise in antibody titer to HA is measured by HI, and is used for diagnosis.
|
|
|
Influenza Replication
|
1. The virus binds to a cellular glycoprotein with a sialic acid bound to a galactose (Via HA/NA).
2. The virus is endocytosed (**rare) 3. The matrix has pores that allow for the influx of Cl- ions, which shift allows for the fusion of the matrix and endosome membranes (reverse donut structure), causing ejection of the genetic material etc. 4. The - RNA is transcribed into + mRNA. Gene/Proteins: The first 3 segments are RNA polymerases (1 and 2 take -RNA to +mRNA, 3 takes +mRNA to -RNA). Next is the NA gene, Nuclear capsid gene (AKA Ribonuclear protein/RNP Type A, B, and C which is used to class the virus), The HA (receptor/vaccine), Matrix proteins, and the non-structures proteins (NS1 and NS2 which are virulence factors interfering with IFN signaling). Note that influenza RNA has not polyA tail and cap, so it "cap snatches" one from host RNA (destroying its function and thus damaging the cell). After viral protein genesis and assembly, NA cleaves the sialic acid on the interior membrane and is necessary for virus exit. |
|
|
Genetic variation for influenza
|
The substrain of influenza (not A/B/C, but subtype of each) is determined by the HA/NA types. This is often determined as a function of the ability of control serum (vaccinated with the current vaccine regimen) to agglutinate the virus. If the new virus is only somewhat agglutinated the old vaccine can still be used, and the alteration is the result of drift (normal slow genetic plasticity).
If the control serum is completely inadequate to hemagglutinate the virus a "shift" has ocured produces a new strain which could trigger an epidemic. In that case the vaccine needs to be reformulated immediately. This is why anyone who dies from the flu needs to be sampled and the strain tested. Reassortment can also trigger epidemics. It occurs when multiple virus strains infect a host cell, however the 8 different RNA segments can end up sorting into different viruses before exit. This is the fear with swine being a host for both human and avian influenza viruses with the possible transfer of morbidity/mortality factors. |
|
|
Respiratory Viral Infections
|
There are 2 general types, Localized in which the virus enters and remains in the respiratory tract causing diseas in either upper or lower RT..or Generalized infection in which the resp tract is the entry point leading to entry into the circulatory or lymphatic system.
Localized upper respiratory tract infectons: Paramyxovirus family (parainfluenza 1 & 2, RSV) Orthomyxovirus (influenza) Picornavirus (rhinovirus) Coronavirus (SARS) Adenovirus Localized Lower respiratory tract infections: Orthomyovirus (influenza) Paramyxovirus (parainfluenza 3, RSV) Generalized Viremia: Paramyxovirus (mumps, measles) Picornaviurs (polio) Poxvirus (smallpox) Parainfluenza 1, 2, and 3; RSV, Rhinovirus, and Influenza acount for the vast majority of respiratory viral disease. |
|
|
Parainfluenza Virus
|
Structure:
Belonging to family paramixovirus, they are enveloped, pleomorphic, with single stranded negative non-segmented RNA. There are 4 serotypes (1, 2, 3, 4) with 1-3 being clinically significant. Additionally the virus carries an RNA polymerase. Biology: The envelope includes HN proteins (Cell receptor) and F (clevage by host enzymes is required for penetration and uncoating). Epidemiology: Parainfluenza 3 is a serous Nosacomial infection while 1 and 2 are seasonal infections of the fall and winter (spikes in Sep-Jan), often in alternate years. 90-100% of kids >5 yr are + for Parainfluenza 3. 75% also have antibodies for 1 and 2. Oftne children are the source of adult infection. All 3 present similarly, so this is an important distinction. Transmission/Pathogenesis: Infection initiation by close contact (reason 4 seasonality), virus entry via mucus membranes or vir touch. Replication in the upper resp tract epithelium, no viremia. Incubation of 2-3 days, shed 8-10-30 days even after the pt is asymptomatic. Immunity: Humoral - little value with no viremia. Secretory- provides some protection, but not in young kids. Passive- significant No vaccine. Clinical symptoms: Croup (hacking cough,Type I and II) Bronchitis (PI III) Pneumonia (PI III) |
|
|
Respiratory Syncitial Virus (RSV0
|
Structure:
A paramyxovirus, it is enveloped, pleomorphic, and has a sing stranded non-segments - RNA Genome. There are 2 serotyped designated A and B. Biology: on the surface of the envelope we have F proteins (Fusion as the virus spreads via cell fusion creating large synctia, hence the name) and RNP complexes which are the host cell receptors. Epidemiology: The primary cause of lower resp tract illness in young kids, often resolving in otherwose healthy kids. Seen frequently in hospitals, day cares, and nursing homes. Spread via close contact, so it is prevalent from Nov-MAr in northern climates, later in warmer location. 50% of all kids are + for AB by age 1. Factores contributing to increased risk of RSV complications include prematurity/age, congenital heart or chronic lung disease (like cystic fibrosis), imunodeficiency lower socioeonomic status/living conditions. Non-breastfed infants, and exposure to cigarette smoke. Transmission/Pathogenesis: Infection via large aerosolized respiratory particles (sneeze/cough), nasal secretions and contaminated surfaces. Viral entry via Eyes/nose with infection in upper resp tract. Incubation in 3-4 days, with viral shedding 1-2 weeks prior to symptoms lasting for up to 3 weeks. Immunity: Cell-Cell transmission via fusion so antibodies are nto formed, and the primary infection is not protective of reinfection. Passive immunity from Mom attenuates the severity, and Secretory IgA is effective against F protein. Clinical Symptoms: Childhood- Lower respiratory ilness (most severe), bronchiolitis including cough, wheeze, increase resp rate and retractions. Also leads to pneumonia/resp distress. In adults it is an upper respiratory ilness resembling the common cold. |
|
|
Rhinovirus
|
Structure and Biology:
Picornavirus, non-enveloped, icosahedral, single stranded, non-segmented + genome. There hundreds of serotypes preventign vacination. 2 unique physical proterties include temperature stability (Stabile at room temp and up, repliates best @ 33 C due to the upper resp tract being a few degrees cooler than core), and pH lability (non-acid stabile) Epidemiology: Children are often infected and are the source of adult infection. The elderly are more seriously infected (lower respiratory symptoms, longer duretion, severity, bed confinement). Causes the common cold in late Aus-Oct and Apr-June. Transmission/Pathogenesis: Direct contact with nasal secretions is the major method. The virus enters teh Eye/nasopharynx and attaches to hose ICAM-1 or LDL receptor on the epithelium. Incubation takes 2-3 days, symptoms apprear in 3-4, and shedding begins 1-2 days prior to symptoms and continues for ~1 week. The infection remanse localized to the upper resp and is self limiting. In the elderly the symptoms are more severe, confining many to bed etc. Already exceeds influenza, with risk factors including COPD, Chronic ilness, and smoking. Immunity: Secretory IgA protects against reinfection for 18-24 months. Cytokins are secreted during infection including IL1-B, IL6, TNF, and Kinins. Clinical Symptoms: Sneezing, Sore throat (1st to appear), Nasal discharge (most bothersome)...The common cold. Lasts 9-11 days, longer in the elderly. Note that only humans and Chimps can catch colds. You can catch a cold >100 times, not from wet socks, cold temp or stress, although the latter can worsen symptoms. Extroverts do secrete less virus and have less severe colds than introverts. |
|
|
Tx of Viral Respiratory Disease
|
In general by the time we see a pt with this it is too late to tx the virus (they have been symptomatic for a few days) so we need only attenuate the symptoms. Treatment includes humidified cool air and inhalation of racemic epinephrin/O2/Bronchodialators as needed. Fever reduction, antihistamines/decongestants and antiinflamatories(glucocorticoids). Vitamin C can be helpful (protects against oxidants released by neutrophils and may alleviate symptoms; deficiency leads to vulnerability), Zinc can reduce the dureation by preventing binding to resp ICAM-1, inhibiting capsid synthesis, and inducing IFN production. If indicated we can use meds:
Soluble ICAM-1: inhibits attachment of Rhinovirus IFN: administered intranasally, prophylactic against natural infections Ribavirin: broad sepctrum inhibiting RSV replication and used in many clinical trials. We can try to prevent spread via contact isolation (gloves, gowns, handwashing, no sharing cupa, glasses, utensils etc). There are no vaccines as yet because 1. Rhinovirus has over 100 serotypes, 2. there are other viruses associated with the common cold (corona virus, human methpneymovirus); and 3. the most common strains change very often (RNA polymerase with no edit fxn). Vaccine development for paramyxoviruses has focued on the F protien. |
|
|
Corona Virus
|
Structure and Biology:
Spiked envelope (hence the name "corona") derived rom intracellular membranes containing a non segmented single stranded capped and polyadenylated + RNA genome (the largest of ane RNA virus). The Genome encodes for the following proteins: Spike protein HE proteins Membrane protein Envelope Protein Nuclsocapsid protein The lack of proofreading by RNA polymerase leads to a very high freq of mutation and rapid evolution. The virus matures in the Golgi, accumulating in membrane bound vesicles which subsequently fuse with the plasma membrane releasing the virus. They cause 1/3 of common colds and SARS |
|
|
SARS
|
In late 2002 Severe Adult Respiratory Syndrome was descrribed in souther China, and spread to Asia, N.A. and Europe. It peaked in 2003 and then tailed off.
Caused by a new Coronavirus (SARS-coV), the incubation period is 3-7 days (up to 10) causing characteristic X-ray changes 3-4 days after onset. The fever with progressive woresening is the most important diagnostic feature of SARS. Following incubation the fever comes accompanied by malaise, H/A, and chills for 3-7 days. Then respiratory dymptoms develop includeing non-productive cough and difficulty breathing. This is followed either by slow recovery or pregressive worsening on 10-14 days and death on day 17-18. The virus itself has 11 open reading frames (can produce proteins) with some similar organization to other corona viruses but 9 of the open frames are not in other coronaviruses. Associated with SARS via antibody tests. In addition to standars resp infection clinical cirteria, the CDC has 2 other cirteria for Dx... 1. Travel within 10 days of onset to an area with current of previously documented/suspected SARS. 2. Close contact within 10 days with a person known/suspected to have SARS. definitive Dx is via Antibody to SARS-coV, detection of SARS-coV directly via RT-PCR (performed 2x), or Isolation of the virus. All done at the CDC. Tx begins with isolation and quarantine. That is where the agreemetn stops. Drugs are coming that may block the protease fxn, but there is no vaccine as a result of antigenic shift (live virus is unusable as a result) |
|
|
Herpes Simplex Virus Structure
|
Enveloped icosahedral dsDNA virus whose genome encodes for over 10 genes and has terminal repeats shared by all herpes viruses. The genome is protected by circular proteins called "torris," and the envelope is studded with Glycoprotein G (GPG; different and diagnostic for HSV 1 [below the waist] and 2 [above the waist]), and Glycoprotein D (GPD; Cellular receptor allowing attachment, inactivating antibodies being preventitive, Identical between HSV 1 and 2). Note that even the catifsh variety looks similar morphologically, but is not genetically related. The virus forms inthe nucleus and derives its envelope (makes it less stable so it requires close contact for transmission) from the nuclear envelope. After leavin the nucleus it remains in the cytoplasm, cell associated being the 2nd reason close contact is required for transmission.
The Genome itself incudes 2 terminal repeats and an internal repeat that is the complement of the terminal repeats, dividing the genome into 2 segments names the long and short unique. The genes include Thymidine kinase (making it susceptable to acyclovir), DNA polymerase (again susceptable to acyclovir), GPD (similar in 1 and 2, GPG (different for 1 and 2, used diagnostically, and intracellular protein 47 (blocks antigen presentation allowing for latency). |
|
|
Pathogenesis of HSV 1 & 2
|
Infection:
The virus requires a break in the skin to gain access tot he inner epithelial layer, or deposition on a mucus membrane. It enters and then forms mucocutaneous lesions. From that initial site it jumps into the unmyelinated axon of a sensory nerve ane moves to a nucleus in the ganglia. There it remains latent and may cause 2ndary infection later. HSV 1 goes to the trigeminal ganglia, and 2 tothe sacral ganglia. With the later we get some tingling after the original lesion has healed. Note that about 75% of infections are asymptomatic but lead to life long latency (the symptomatic infections also lead to latency). Later in life reactivation infection can occur as a result of trauma, UV rays, stress, worry, guilt, etc...anything that attenuates the immune system. Note that the primary infection is characterized with IgM, however many may have antibodies that never have developed lesions. Women seem particularly susceptable to recurrance, however acyclovir works well to stop the virus. Also we can check moms to see if their infection is primary (IgM) or 2ndary (IgG). In the former case we definitely want to do a C-section to prevent spread to the fetus. Note that some individuals can shed the virus even in the asymptomatic state. Herpes simplex can cause Encephalitis (including a monkey herpes variety that can infect humans), afect neonates, progressive mucocutaneous infections, infect genitals (primary or recurrent), labia, and cause keratitis. |
|
|
DX of HSV 1 & 2
|
With symptomatic infections, lesions from the 2 appear identical. Lab test can confirm the presence of HSV in lesions and characterize type and weather the infection is primary or secondary. This is done using fluorescent antibodies to HSV which uaually reveal diagnostic Giant cells (syncitia). We can also culture the virus and do antibody checks. we are tending to see more HSV 2 today than 1, as more people are reaching the age of sexual activity without having been exposed to HSV-1..the antibodies resulting from which are protective due to GPD homology.
|
|
|
Treatment/Management of HSV 1 & 2
|
The severity of te lesions tends to diminish over time, and antivirals targeting thymidine kinase and DNA polymerase have been quite effective (acyclovir, although some resistant strains are emmerging). This homology also explains why those previously exposed to HSV-1 did not show improvement wthn vaccinated against HSV 2, they were alredy immune while those with no earlier exposure did see results (the glycoproteins being main vaccine targets, although there is NO vaccine today.
For most infections (except Keratitis for which we use frifluorothymidine) the recommended tx is acyclovir, as well as for prophylaxis in high risk groups. |
|
|
Herpes Viruses (list)
|
Family: Herpesviridae
Subfamilies: Alpha, Beta, and Gamma Alpha: Simplex viruses- HSV1, HSV2, Herpes Virus B Varicella Viruses- Human Herpes Virus 3 (HHV3/Varicella-Zoster virus/VZV) Beta: Cytomegaloviruses- Human Herpes virus 5 (HHV5/CMV) Roseola Viruses- Human Herpes Virus 6 (HHV6/Human B lymphotropic Virus), Human Herpes Virus 7 (HHV7) Gamma: Lymphocryptoviruses- Human Herpes Virus 4 (HHV4/EBV) Rhadinovirus- Human herpes virus 8 (HHV8/KSAHS) |
|
|
EBV associated Infectious Mononucleosis (IM)
|
Epidemiology:
Commonly the infection is uneventful in early childhood, before the immune system is fully competant. IM is the result of a later primary infection with EBV. Pathology: It is the overreaction of the immune system that causes the symptoms of IM. Initial infection is via cell associated or free virus contacting the oral mucosa. Epithelail cells are first infected (sore throat), and then it spreads to the MALT and then B-cells via cd21 (AKA complement receptor 2). The virus causes the manufacture of LMP1 (constitutively active TNF/Oncogene), LMP2 (constitutive BCR), and EBM1 (transcriptional transactivator). These effects make infected B cells PRIME antigen presenting cells and trigger a huge immune response. As a child the immune response is not so severe, but clears the infection, as an adult the response is BIGGER. 1:500 cells in the blood are infected, but most are atypical monocytes/cd8/cd4 cells. There is a permanant scar left on the immune system creating a higher risk for hodgkins disease, and persistance of EBV (in memory cells). Cellular immunodefciency caused by age, pregnancy, AIDS, etc..can bring on fatal mononucleosis later. Ntoe that free virusis made in epithelial cells allowing reinfection, and that in persistant cells the DNA is not integrated by stuck to the chromosome (called an episome) Dx: Signs and symptoms- Malaise, VERY sore throat, Swollen lymph glands. The presence of atypical lymphocytes in the blood is diagnostic, they are mononuclear blast cells (less nucleus and more cytoplasm than fully differentiated lymphocytes), which may be surrounded by a "Dutch Skirt (RBC's stuck around the periphery)." + Mono-Spot test- examination of weather antibodies int eh blood will agglutinate Ox/Horse RBC's (heterophiles). Rise in antibody to the Viral Capsid Antigen (VCA) with IgM indicative of primary infection. Antibody against EBNA (an internuclear EB factor) is indicative of infected cell lysis and coming resolution, and EA antibody spikes and drops normally, however a lack of depression is indicative of persistant infection. Tx: Generally this is a self limiting disease with Tx restricted to SEVERE IM. We can try steroids, however that can be like throwing gas on a fire. |
|
|
EBV associated lymphoproliferative syndrome (Post Transplant Lymphoporliferative Disease-PTLPD) and Non-Hodgkins Lymphoma-EBV-B cell associated
|
Pathology:
Defects in cellular immunity (genetic, aquired, or induced), can lead to uncontrolled proliferation of EBV infected B cells in primary or reactivation infection. Dx: Id of monoclonal/polyclonal proliferation of EBV carrying B cells. High levels of EBV DNA (remember it is an episome so it proliferates when its B cell does), and/or antibodies to EBV. NHL-EBV-B cell is visualized via anti-LMP1 antibodies. It is not a true tumor, but as it can't be killed it acts like one and requires aggressive therapy. Tx: Requires aggresive therapy including "adoptive immuno therapy) |
|
|
EBV associated Tumors
|
In general can be caused by cellular immune response defects.
Sporadic and Endemic Burkitt's Lymphoma: Requires 2 hits, one being presence of EBV and the other a genetic change in c-myc oncogene(8:14 reciprocal translocation placing an IG promoter upstream of c-myc turning it on ALOT. C-MYC stimulated DNA synthesis makind this a real tumor). The 2nd hit is possible due to all of the genetic changes in B cells (class switching, VDJ joining, Somatic hypermutation, etc). Nasopharyngeal Carcinoma: Clusters geographically (SE Asia and elsewhere) and is 100% associated with EBV. Hodgkins Disease- The incidence is higher in individuals that have had IM, and some types of HD tumor cells are infected with EBV. The tumor has mixed cellularity, but the lymphocyte predominant form is most common. Classic Reed-Sturnburg cells have a hodgkins tumor cell in the middle surrounded by fibroblasts, stromal cells, monocytes, and MANY lymphocytes. The center cell has all the hall-marks of a B cell (VDJ, Class switching, somatic hypermutation) however the receptor is not functional. Normally that cell would have apoptosed, however the presence of LMP 1 and 2 (a result of EBV) prevents apoptoses so the cell becomes a tumor. |
|
|
Cytomegalovirus (CMV, HHV 5)
|
Large ubiquitous virus.
Latency is in the common precursor to macrophages and dendritic cells (CD34+ 13+). The virus replicates when the cells do (along either line), driven by inflammation. Thus the big problem is in transplant patients. Epidemiology: Most infections are early in life and are asymptomatic. More people today are reaching adolescence and adulthood without being exposed, and thus they get sick. Transmission: With infection the virus is excreted in the urine and thus it aerosolized and spreads through the childs school/home. It can also be transmitted via saliva, sex, or blood. Tx: CMV is a lytic virus and replication can be inhibited by nucleoside analogs such as gancyclovir. |
|
|
cytomegalovirus induced Atypical Mononucleosis
|
Very similar to EBV Infectious Mono.
Similarities include: Atypical lymphocytes Lymphadenopathy Malaise Etc... Differences include: No sore throat Negative Monospot test Dx is via rise in Ab titer to CMV in the long term, and inclusion bodies in uring spun down, or virus isolation. |
|
|
Congenital Cytomegalic Inclusion Disease
|
A disease of newborns infected with CMV in utero. It most often involved a CMV primary infection during pregnancy (as opposed to latency). CMV- Pregnant women most at risk include teachers, nurses, etc.
The fetus may be symptomatic, or asymptomatic. Clinical signs include petichiae, hepatosplenomegaly, jaundice, microcephaly, premature birth/small gestationsl age, mental retardation, hearing loss, learning disabiliteis, and dental defects. Note that the placenta is also infected at this time which -'tively effets gas/nutrient exchange. The difficulty here is that many initially infected moms are asymptomatic, thus we need to monitor her blood and may give immunoglobulin. Note that those who are CMV+ pre-conception can still infect the fetus if they have a peripregnancy reactivation, but symptoms are rate. |
|
|
Cytomegalovirus in the Immunocompromised Pt.
|
Normally asymptomatic infection/reactivation in an environment of reduced cellular immunity can be life threatening. Often this is seen in transplant patients (remember that inflamation triggers proliferation).
The donor may be initially CMV + or - (most are +). The recippient is also either + or -. CMV + organs cannot be given to a CMV - recippient. There can also be problems with a CMV + recippient. When the virus reactivates it can go the the eye, lung, kidney, etc... causing CMV disease. Note that virus from either source is reactivatable. Tx is via gancyclovir which works b.c in the long uniqe there is a thymadine kinase (gene Long 97). A CMV recippient NEEDS a CMV - organ. The same problems exist with blood transfusions. |
|
|
Varicella Zoster Virus (VZV)
|
Chicken Pox- A very infectious disease in youth resulting from primary infection with VZV. Initally it spreads through cough, but in the later stages skin lesions are infectious, and it generally is symptomatic. It is becoming less and less frequent as a result of the vaccine. Still not harmless in kids, leads to 1000's of hospitalizations and 50-60 deaths/year, along with many complications. Can be especially nasty in neonates (Infected via Mom).
Zoster/Shingles- Disease of adult expression of VZV (almost always a result of reactivation of latent virus, but primary infection is possible). VZV remains latent in the CNS so that reactivation si very painful and lesions are often along particular dermatomes corresponding to the infected nerve. Leesions are infectious, but not to the same degree as chicken pox. Treatment- Zoster Immune Globulin: Pooled sera from Zoster patients used prophylactically in susceptable patients exposed to VZV. Oka Vaccine Strain: Serially passages virus becomes attenuated and provides long lived protection in 80% of people. A booster is also helpful. vaccinating the elderly devreases the incidence of shingels by 50%. Note that the vacine was not originally offered universally as they were not sure that protection would be permanant and wanted to prevent shifting the disease into adulthood. |
|
|
Human Herpes Virus 6 (HHV6)
|
Causes Roseola Infantum (aka Examthem Subitum, 6th Disease) in children. The disease is generally mild and self limiting. Rarely cases of Ifectious Mononucleosis may be associated with HHV6. Note that it was tough to isolate (finally done using an HIV pt).
|
|
|
Human Herpes Virus 8 (HHV8)
|
Also known as Kaposi sarcoma Associated Human Herpes Virus (KSAHHV). Originally discovered in Africa and later re-surfaced in AIDS patients. It is endemic to Africa, but today we see 4 kinds of patients with KS, Classical, Equatorian African, AIDS, and Transplants. Originally, it was also noted that the lesions dissappeared 50% of the time.
Characterizatin of the virus occured when it was noted that MSM HIV patients got KS far more than IV Drug Users, so a differential display gel was run to compare DNA and the Viral cause of KS was revealed. Like Hodgkins disease, tumors are of mixed cellularity with a central HHV8+ cell surrounded by fibroblasts, endothelial cells, lymphocytes, etc. The tumor secretes VEGF causing blood vessles to grow into it (it is angiogenic). HHV8 can also casue Body Cavity-based Lymphoma (AKA Multi-centric Castleman's Disease). |
|
|
Adenovirus (morphology/Genome/Replication)
|
Morphology/General:
There are 2 genera (Aviadenovirus (birds) and mastadenovirus (mammalian) and 7 subtypes (A-G). The clinical spectrum varies with both subtype and method of transmission (e.g. Type 7 inhaled is a severe lower resp syndrome, oral transmission is mild to asymptomatic). They are non-enveloped icosahedral viruses with a non-segmented linear DS DNA genome. The capsid consists of Hexons interspersed with 12 pentons to which the fibers with cell receptors are anchored. The latter has a toxin-like cytopathic effect. Inside the capsid are at least 10 proteins (labeled II - IX and TP with no I or X. Genome: Linear Non-Segmented DS DNA which may encode 30-40 genes. Structure/homology is used to assign to groups. The terminals have inverted repeats, and there is a protein covalently linked to the 5' end of each. Replication: Ocurs in the nucleus and is divided into early and late phases with DNA replication signaling the transition(characteristic of DNA viruses). 1. Attachment- Slow, 2 stage process. First the fibre interacts with one of a number of cellular receptros including MHC I & Coxsackie-adenovirus receptor. This is followed by the interaction of the penton base with an integrin causing receptor mediated endocytosis. Most cells have primary receptors but internalization is more selective. 2. penetration- Phagocytosis followed by rupture of the vesicle via penton activity and release into the cytoplasm. 3. Uncoating- Ordered, 1. Pentons, 2. partial uncoating of DNA, 3. Migration to nucleus, 4. conversion to Viral DNA-Histone complex. 4. Expression- Immediate early genes first (E1A- first to transcribe, transactivator which is necessary for early genes), then early genes (E1B, E2A, E2B, E3, E5, Some virion proteins). Note that genes are encoded in various location on both strands of the DNA. 5. DNA Replication- requires 3 proteins...TP (promer for initiation of synthesis), and DBP (DNA Biding Protein), and Ad DNA Pol (DNA Dependant Polymerase). 6. Late gene expression- Starts at the onset of DNA replication, includes virion proteins, and only uses Newly replicated DNA!!! 7. Assembly- Begins in the cytoplasm, but occurs in the nucleus. Infected cells do not lyse, but round up due to cytoskeletal changes. Virions accumulate in the nucleus and are visualized as inclusion bodies, thought to be the basis for altent infection (called occult/hidden infection). |
|
|
Adenovirus Proteins (in the capsid)
|
Name Location Fxn
II Hexon Monomer Structural III Penton base Penetration IIIa Assc /w base Penetration IV Fibre Binding/HAG V Core (DNA & Histone-like Base) VI Hexon minor Ass/Stabile VII Core Histone VIII Hexon Minor Ass/Stabile IX Hexon Minor Ass/Stabile TP Genome Term Replication protein Mu Core Unknown |
|
|
Adenovirus Gene products/Functions
|
Immediate Early and Early:
E1A- First made, can immoralize cells in vitro...binds RB protein to prevent tumor supression. E1B- 2nd product made, binds to P53 to prevent tumor supression and allow progression of the cell cycle. (note that this kind of mechanism is used by many DNA viruses). E1A and E1B together are necessary for full transformation and tumor formation (only happens in animals). Transformation is just a side effect of viral replication which is the real goal (Transormation = Change in morphological/biochemical, or growth peramaters of the cell which may or may not include neoplastic transformation). E3- Downregulates MHC I antigens, Inhibits lysis by TNF, and Apoptosis by Fas, thus reducing immunogenicity and possibly facilitating persistance |
|
|
Adenovirus (Pathogenesis, Transmission, Clinical course, Dx, Tx)
|
Pathogenesis:
In general, most infections are asymptomatic and are common, most people have been infected with at least 1 type by age 15. It is widespread in nature infecting birds and mammals, it can be latent in lymphatic tissues and may undergo reactivation. Several kinds (esp Type I) are oncogenic in animals. In closed quarters/military/boarding schools: Acute respiratory illness, Pneumonia, Conjunctivitis/Keratoconjunctivitis. In Infants: Pharyngitis, Gastroenteritis, Pneumonia, Acute Hemorrhagic Cystitis, Hepatitis, Conjunctavitis/keratoconjunctavitis Liver Transplants (Hepatitis) Transmission: Fecal/Oral, Respiration/droplet, Hand/Eye contact, Venerial. Note that the virus may be shed for weeks/months after initial infection, and as it is resistnat ot chemical and physical agents including pH it may survive for prolonged periods outside the body. Clinical Course: Incubates in 5-8 days, and usually causes localized infection self limiting within 2 weeks. Imunocompromised patients may get generalized infections. Latency is in lymphoid tissue and kidneys (the majority of tonsils removed are adeno+). It is endemic in pediatric populations and may cause acute respiratory ilness, Meningoencephalitis, and death if Adenovirus 7 is involved. Dx: Via enzyme immunoassay (EIA), Immunofluorescene, PCR, and isolatin in cell culture (visualize cytopathic effect (rounding up, swelling, basophilic inclusion bodies) Tx: Antivirals are generally ineffective but IV ribavirin does have potential. As the disease is so mild most of the time, only military personnel are vaccinated wtih the live attenulates oral virus resulting in mucosal and intestinal immunity. (Adolescents and others in close daily contact are at risk for epidemic spread of respiratory infections, but the risk of oncogenic potential limits the desirability for widespread use). Infection ledas to long lasting immunity against specific serotypes, and maternal antibodies are protective. The disease is preventale with chlorination of water (pools, waste, drinking, etc), hygene in opthamology/hand washing, and measures to prevent nosocomial infection. |
|
|
Factors that made Smallpox eradicable
|
1. A severe disease with morbidity and mortality
2. Considerable savings to developed non-endemic countries 3. Eradication from developed countries demonstrated its feasibility 4. No cultural or social barriers to case tracing and control 5. Long incubation period 6. Infectious only after incubation period 7. Low communicability 8. No carrier state 9. Subclinical infections not a source of infection 10. Easily diagnosed 11. No animal reservoir 12. Infection confers long-term immunity 13. one stable serotype 14. Effective vaccine available |
|
|
Smallpox
|
Morphology:
This is the largest and most complex virus. Oval or brick shaped, can just be viasulaized by the best light microscopes. External surface is ridged in parallel rows, and can be helical. They contain over 100 proteins. The extracellular forms have 2 membranes (EEV = Extracellular enveloped virios) whereas the intracellular form has only an inner membrane (IMV-intracellular mature virions). The outer surface is composed of lipid and protein, the core is biconcave (dumbell shaped) with 2 lateral bodies with tightly composed nucleoprotein in the center. The virus also has at least 10 enzymes (mostly for Nucleic acid metabolism/genome replication). It is antigenically comples inducing specific and cross reacting antibodies (henve the vaccine) Genome: Linear DS DNA with a terminal hairpin loop (no free ends) with several tandem direct repeats. Most of the essential genes are in the middle, it has a total of about 250 genes. Replication: Occurs in the Cytoplasm (** odd for a DNA virus, but this one has everything it needs) 1. Adsorption- The receptors are not known, but there are likely multiple receptors on different cell types. Vaccina likely uses the Epidermal Growth Factor (EGF receptor) 2. Penetration- Complex, possibly multiple mechanisms 3. Uncoating- 2 stages, removal of the outer membrane upon entry to the cell, and then full uncoating in the cytoplasm. 4. Gene expression- Uses viral enzymes associated with the core to express the early genes (50% of the genome, pre-replication) and then the late genes (after replication, dependant on replication for activity). Vaccine is resistant to Interferons as one of the ealry genes and one of the late genes inhibit PKR activation. Others interfere with the actions of complement, IL1 an dTNF's. Vaccina infection of cells can confer protection from IFN on other viruses. 5. Genome Replication- May involve self-priming. The many viral enzmes used for replication are potential drug targets (including Thymdine Kinase) 6. Assembly- Occurs in the cytoskeleton. Actin comet tails form which shoot IEV through the cytoplasm to the surface and perhaps to adjascent cells. pathogenesis: Tranmitted via respiration of lesion material in resp tract of pt's in the early stages of disease. During the 12 day incubation, it is distributed to the internal organs, and then to the skin. Management depends on isolation of infected individuals and vaccination of clsoe contacts. If given during the incubation perior it prevented or reduced the severiyt of clinical symptoms |
|
|
Adenovirus Interaction with other viruses.
|
Adenoviruses are known to interact with other viruses (esp parvovirus AKA Adenoassociated viruses, and SV40 AKA Papovirus). The latter acts as a helper to overcome late blocks to adenovirus replication in some cell types. Hybrid formaiton is also possible indicating functional overlap between the 2 families.
|
|
|
Variola and Vaccina
|
> 9 different pox viruses cause disease, but variola virus (VV) and Vaccina are the best known. VV is divided into variola major and Minor, the former having much higher morbidity/mortality.
variolation- Administration of material from known smallpox cases (variola Minor) to protect recipients. It is risky though. The vaccine strains (vaccinia) have been propogated for many years, and has been distinct from cowpox (one possible origin) for at least 50 years. |
|
|
Smallpox (typical disease progression)
|
7-17 days (12 avg) incubation
2-4 days of fever macules- rash emerges as red spots on tongue and mouth which develop into sored which open and spread lots of viru sinto mouth (highest contagion point 7-10 days). At this time the rash appears on the skin, starting on the face, to the arms and legs, then hands and feet. full coverage in 24 hours papules- by the 3rd day of the rash, raised bumps (papules) appear. vesicles- By day 4 bumps fill with thick opaque fluid and have a central depression (distinguishing characteristic of smallpox) Pustules- the bumps become pustules (sharply raised, firm) which are contagious Scabs- the pustules begin to form a crust and scap over. By the end of the 2nd week of the rast, most have scabbed...the pt is still contagious. They begin to fall off becoming pitted scars. The person is no longer contagious when all the scabs are gone. |
|
|
Monkeypox
|
Rare viral disease originally found in monkeys, reported in humans in 1970. It is an orthopoxvirus (like smallpox (variola), cowpox, and the smallpox vaccine (vaccinia). The clinical disease is identicel to smallpox. In 2003 some folks in the US got it from prarie dogs. It has been suggested that the relenting of the smallpox vaccine campaign has led to its immergence as vaccinia is also protective for monkeypox. Recently we have seen the rate of person to person transmission jump, however the proportion of deathd did decrease. CHildren in frequent contact with animals are most at risk.
Pathogenesis: Zoonosis from monkeys via bite, contact with blood, body fluid, or rash. Also person to person via resp. droplets (in prolonged face to face contact), touching body fluids, blood, or contaminated bedding/clothing. Symptoms: Similar to smallpox, but generally milder. Another difference is that monkeypox causes lymph nodes to swell. It includes Fever, Headache, muscle ache, backache, lymphadenopathy, malaise. After onset of the fever they will get the rash. The ilness usually lasts 2-4 weeks. Mortality: In aftica, 1-10% died, but that would likely be lower in the US. The risks from monkeypox disease are greater than those from the smallpox vaccine, so that prophylaxis is desirable. Tx: Preventitive via timely pre/post exposure use of the smallpox vaccine. It is at least 85% effective in preenting monkeypox, both preexposure, and it is also good if given <4 days post exposure. If it is given 4-14 days post exposure, it may still attenuate the symptoms if it does not prevent the disease. Prophylactic vaccine is indicated for those who are exposed or are likely to be exposed, however NOT immunocompromised individuals, or people with allergies to any part of the vaccine (Latex, Polymixin B, Chlortetracycline, Neomycin). Note that if you are exposed to monkeypox, and it has been >3 years since your last smallpox vaccine, you need to get one ASAP. |
|
|
Similarities between Measles, Mumps, and Rubella
|
Humans are the SOLE reservoir
Transmission is by person-to-person contact via the respiratory route All three diseases are preventable by the use of specific, live attenuated viral vaccines Distribution is world-wide, with a high incidence of infection in susceptible individuals Distribution has shifted in recent years from school age children to pre-school children and young adults Change in distribution reflects the widespread use of effective vaccines Susceptible population consists of those individuals who were never vaccinated and those who were improperly vaccinated |
|
|
Mumps
|
Biology/Epidemiology:
Outer envelope containing hemagglutinin and Neuraminidase (HN is the receptor, F glycoprotein must be cleaved for penetration), and there is only 1 serotype. In vitro growth is best indicated via hemadsorptin as CPE is variable. World wide distribution. Person to person spread via direct contact, resp secretions, saliva, and possible urine. 85% in kids <15, with epidemics more common in the late winter/early spring. 30-40% of infections are subclinical. Also note that infections in children <6 mos is rare as a result of Mom Immunity. Pathogenesis/Disease: Any infection produces life long immunity. The virus initially infects the resp tract and then viremia carries it elsewhere,, the salivary and other gland beng the most susceptable. Incubates in 14-24 days and causes painful enlargement (often unilateral) of the salivary glands. The swellin peaks in 3 days and subsides in 7, with moderate fever in 80% of cases. Complications include- Meningocephalitis- most frequent compliation with H/A, N/V, Irritability and rarely onvulsions. moderate neck stiffness, high cells, low glucose. Other problems include Orthitis/Epididymitis/oophoritis, pancreatitis, nehritis, Thyroiditis, Myocarditis, Arthritis, and mumps embryopathy Dx/prevention clinically made using symptomology/exam. In the lab (if parotiditis is absent), we an isolate the virus from saliva, urine, and CSF. Serological DX is via antibody titers to the Viral (V) and/or soluble (S) antigens early in the disease. Live attenuated vaccine produced in chick embryos is available alone or as the MMR combo. Recommended fo rkids over 15 months, and adult males with no Hx of infection. |
|
|
Measles
|
Biology/Epidemiology:
One serotype, grows in culture and forms giant cyncytial cells wtih intranuclear and cytoplasmic inclusion bodies. World wide distribution, it spreads bis respiratory tract secretions and perhapsurine. Most are infected <6 years old, and epidemics were in late winter/early spring before the vacine. Rare in kids <6 mos due to MOM immunity. Initial replication occurs inteh respiratory tract and is followed by viremia, with lesion formaiton in the skin (exanthem), and mucus membranes of the pharynx (Koplik's spots). 3 stage cycle, 1-incubation for 10-12 days, 2-Prodromal stage wtih Koplik spots, mild/mod fever, slight conjunctivitis, coryza and increasingly severe cough, and 3-maculopapular rash all over with high fever. Max contagiousness is via droplet spray during the prodroma stage, contagious from 7 days s/p exposure through the first few days of rash. Clinical Disease: Koplik Spots are the DX sign, conjunctivitis, photophobia, and fever are also comon. The rast starts as faint macules which progress to maculopapules. Chief complaints include otitis media (2ndary bacterial infection), Pneumonia (may cause interstitial/Giant cell pneumonia, but pneumonia 2ndary to superinfection is more common. It may exacerbate TB however, and may cause a temp loss of hypesensitivity to tuburculin, and may exacerbate TB. Most common measles problem is encephalitis (1-2/1000). It also causes subacute sclerosing panencephalitis. Dx/Prevention Isolaton from nasalcavity, throat, eyes, and urine. CPE (Cell pathogenic effect?) consists of both nuclear and cytoplasmic inclusion bodies. Histo reveals giant cells. Killed vaccine is no longer available b/c of reactions in immunized kids with later exposure to WT virus. Live attenuated vaccien is available and can be alone or in concert with MMR. The vaccine is not recommended in kids <15 months. |
|
|
Rubella
|
Biology/Epidemiology: Agglutinats mammalian and avain RBC's, no CPE apparant in th lab, with frowth detected via the intererence technique using n enterovirus. Peaking in he late winter/early spring, world wide distribution with epiemics every 3-4 years. It is rare in kids <6 mos due to Mom immunity. Tranmission is via nasopharyngeal secretins or infected urine (droplet ro direct contact), although it is not highly contagious. Many individuals escape childhood infection, but may be susceptable as young adults.
Clinical Disease: Incubates over 14-21 days, with communicability 7 days before symptoms to 5 days after rash appearance. Initially replicates in cervical lymph nodes (Not resp tract like measles or mumps), leading to viremia (7-14 days, at which time antibodies are first detected. The development of Ab coincides with appearance of a maculopapular. Dx is via isolation form urine and nasopharynx. The disease is mild including rash, lymphadenopathy, low fever, and transient arthralgia/arthritis, esp in adult females. Complications are rare and include thrombocytopenia or encephalitis. HX of disease does not indicate definite immunity, but once it is there itis life long as there is only one type of virus. Congenital infection (Mom gets it in the first trimester) can cause mental retardation, deafness, cataracts, heart defects, Microcephaly, hydrocephaly, hepatitis, pneumonia, arthritis, chronicn meningitis, thrombocytopenic purpura, and bone lesions. Sub-clinical maternal infection is therefore BAD, as we prevent more stuff the earlier we catch the bug. Also, the later mom is infected, the less chance there is of fetal malformation. Congenitally infected infants will excrete virus in the nasopharynx, Urinary tract, spinal fluid etc...for months-years. The infants have high Ab titers despite chronic infection, and are an important source of infections. Dx/Prevention: Difficult to Dx as the rash may look like adenovirus/enterovirus. Isolatin and serologic diagnosis via neutralizatin, complement fixation, hemagglutin inhibition or fluorecent antibody tests are used. If congenital, the cirus can be isolated from numerous fetal tissues, placents, amnionic fliud, et. Neonatal specific IgM can be demonstrated by immunofluorescence, even in the presenceof passive aquired IgG. The Live attenuated vaccine can be used alone or as MMR. Should be given to kids >15 months, and women who are seronegative and not yet pregnant. It MUST NOT be given during pregnancy. |
|
|
MMR vaccine
|
Trivalent for measles, Mumps, Rubella, all life attenuated strains. Induces serum antibodies in 95% of patients, and recommended for all infants 12-15 months of age. Mom immunity may interfere if it is given to a child <1 Yr old. Booster at age 4-6 (just prior to entry into school) is recommended.
Not eligable for vaccination: Immunodeficient patients patients with acute febrile illness Patients haveing recieved immune serum or a blood transfusion within 3 months patients with a known anaphylactic rxn to eggs (M and M in chick embryos). Females of child bearing age unless Pregnancy is ruled out and will be avoided for >3 months. Indication for revaccination: Vaccinated before first B-day Vaccinated with killed measles vaccine Vaccinated prior to 1968 with an unknown type of vaccine Vaccinated with IG in addition to a further attenuated strain or vaccine of unknown type |
|
|
DNA Cancer Viruses (similarities and general characteristics)
|
Papomaviruses are a family of small non-enveloped covalently closed circular DS DNA viruses. They cause progressive neurological disease as well as papillima's (warts). They are also associated wtih cellular transformation in animals an dcervical carcinoma in Humans. There are 2 genera;
Papillomavirus- responsible for warts in humans Polyomavirus- causes tumors in animals and progressive multifocal Leucoencephalopathy (PML) in man. Their genes can be divided into: early- Regulatory in nature, AKA T antigens, Expressed imediately, regulate both viral and cell fxn. late- structure in nature, expressed only in a permissive infection in which viral progeny are produced after replication has begun. Inlude capsid proteins. |
|
|
Papillomaviruses
|
Unique Characteristics:
Larger genome and are larger in size. Over 100 types based on DNA homology (<50% homology = new type). Extremely species specific, causing fibroepitheliam and squamous epithelial tumors ONLY in their natural host (from which they are isolated). Tough to grow in culture (require highly differentiated keratinocytes) To cause a wart: 1. The steps from Adsorption to viral DNA replication all occur ONLY in the (dividing) basal and suprabasal epithelial cell 2. The Remaining Viral DNA replication and late gene expression through release of virla particles ONLY occures in terminatllly differentiated (non-dividing) keratinocytes. To transform a cell, HPV16 and HPV18 use 2 early gene products E6 and E7, both of whcih are required: 1. E6 binds and causes degradation of P53 (tumor supressor) which leads to decreases in levels of WAF-1 and allows progression through G-1 cell cylce. 2. E7 binds to and inactivates retinoblastoma protein, also a negative regulator of cell growth. This disinhibits E2F1, allowing the transition from G1 to S phase, transcription of DNA synthesis genes, and progression through division. Principals of Papillomas: Individuals most at risk of papillomas include institutionalized individuals in frequent close contact, and immunocompromised folks. All infections are Localized and result after LONG incubatin periods (freq. >3 months). The most common exhibit frequent spontaneous regression, and recurrence, the former a result of cellular immune responses to infection, and the latter determined by humeral immune response (pt's with competent IgM and IgG are more likely to be free from recurrence than those with only IgM). Oncogenic transformation is common, HPV associated with genital tract lesions are thus divided into high risk (Types 16 and 18) and low risk (Types 6 and 11) based on the likelyhood of malignant progression. |
|
|
Common Wart
|
Properties:
Occur singly or in multiples, often on the hand of young kids. Virus: HPV 1, 2, 4 Transmission: Direct contact or via clothing, jewelry, etc. Penetration: Requires break in deal layer of skin Organ infected: Skin Incubaton Period: >100 days Clinical Significance: Common, superficial/cosmetic Malignant Conversion: Rare Tx: Freezing, Chemical removal, for cosmetic purposes only. Regression occurs normally in 25-35% in 6 months, 35-70% in 2 years. |
|
|
Flat (Plantar) Wart
|
Properties:
Occur singly on sole of foot (kids), or multiply on face and other extremeties (excluding hand and foot) Virus: HPV-3 Transmission: Direct contact with moisture and pressure point injuries. Common in kids, adolescents, and users of communal facilities (pools) Penetration: Requires a break in the skin Organ Affected: Skin Incubation Period: 30-100 days Clinical Significance: Nuiscance, but may be painful if deep. Malignant Conversion: Rare Tx: Freezing for cosmetic purposes, normally regression occurs. |
|
|
Epidermodysplasia Veruciformis
|
Properties: General degredatin of flat warts leading to malignant conversion
Virus: HPV-5 (also 3, 8, 9..5 is most important) Transmission: Initially by direct contact then genetic (Virus maintained as an episome passed from parents to offspring as an autosomal recessive disease) Penetration: Skin break required for initial infection Organ Affected: Skin Incubation Period: >100 days, variable with genetic infection Clinical Significance: Malignant conversion in areas exposed to sun. Malignant Conversion: 25-30% of all cases Tx: surgery indicated due to liklyhood of cancer. |
|
|
Genital Wart (Conduloma Accuminatum)
|
Properties: Occuron mucoas surfaces of external genitalis and perianal region.
Virus: HPV-6, 16, 18 Transmission: Sexual Contact (65% infectivity) affected by hormone status (pregnancy) Penetration: requires skin break Organ Affected: external genitalia/perianal area Incubation Period: 3 mos-2 years Clinical Significance: Freq regress in females and then occur wtih increased size during pregnancy Malignant Conversion: Precursors to squamous cell carcinoma of the cervix (84-96 months) Tx: Surgery is indicated due to the liklyhood of cancer |
|
|
Laryngeal Warts
|
Properties: Flat/slightly elevated multiple benign tumors of the larynx and vocal cords
Virus: HPV-6 (same as genital) Transmission: During passage through birth canal in moms with genital warts (? oral sex??) Penetration: None required Organ Affected: Exposed epidermal cells of larynx Incubation Period: 60-90 days Clinical Significance: Serious problem for infants due to airway obstruction. Malignant Conversion: rare but possible Tx: surgery to restore airway, however they may recur as frequently as 3X/Year |
|
|
Polyomaviruses
|
Unique Properties:
12 types, BK, JC and SV40 being the best studied. All share considerable seq homology and common antigenic determinants. Note that of the 3, SV40 does not infect humans. They are specied specific, however unlike papilloma viruses, they DO NOT cause tumors in their natural hosts. Grow easily in culture, they are good to study. SV40 was the first virus genome to be mapped via restriction endonucleases and completely sequenced. They can infect a wide variety of celltypes, but the result may be productive or non-productive. Some polyomaviruses are only capable of being productive in a narrow range (JC replicates ONLY in human fetal flial cells). In the case of a non-productive infection (Viral DNA replication does not take place), Early Viral mRNA's are produced stimulating the cell to progress through the cell cycle, however if viral large T antigens are not present in high enough quantity, or if they cannot interact with the appropriate cell replication factors, viral DNA is not replicated and no progeny are made (the large T controls this step. Expression of the large T antigen however does alter growth properties of the cell. This is generally short lived as the viral genome is lost, however if it integrates into the host genome it will beceome constitutiely expressed and transform the cell permanantly. Large T is in SV40, Middle T is in polyoma. Large T acts like HPV E6/E7 bindign to an inactivating both p53 and RB. Middle T resembles an activated growth factor receptor. It locates on the plasma membrane interacting with signal transducers constitutively, allowing proliferation |
|
|
Polyoma Tumor
|
Prototype polyomairus producing histologically distinguishable tumors in newborn rats and hamsters (not in adults of mice, its natural host)
|
|
|
SV40 Tumor
|
Causes tumors in newborn hamsters but not monkeys, its natural host. First isolated from primary monkey kidney cells used to propogate polio virus for vaccination. May be present in human tumors of the choroid plexus.
|
|
|
BK/JC Tumor
|
Widespread in humans (most adults have antibodies) infecting in early childhood. Primary infection is asymptomatic, but is followed by a low grade persistant infection activates when the immune system is compromised. Both are usually established in the UG tract. BK can be isolated from the urine of renal txplant patients. JC caninfect oligodendrocytes of the brain and cause Progressive Multifocal Encephalopathy (PML). Both have oncogenic potential as they have been shown to induce brain tumors in a number of animals.
|
|
|
RNA cancer viruses (General Info)
|
General Characteristics: Cause leukemias and solid tumors as well as aids and specific T cell leukemia. Enveloped SS RNA X2 (THE ONLY DIPLOID) viruses. They are divided based on their means of transmission and types of cells they infect as endogenous and exogenous. The most distinctive difference between the 2 is how they form tumors.
Endogenous: Leukemia/Leukosis viruses infecting the GERM LINE cells and integrating thie genetic information in. The result is a proviurs that is inherited as a dominant genetic trait, whose expression is regulated by those factors that influence cellular genes. They do casue tumors but VERY SLOWLY, Occuring late in life after a prolonged latent period. These cells can be induced to release the virus via exposue to carcinogens. Exogenous Retroviruses: Sarcoma/Acute leukemia viruses causing many tumors with short latent periods. They integrate into somatic cells, and thus are not passed vertically through generations. The major differences betwen endogenous and exogenous retroviruses include the cell type in which they integrate, and the mechanism by which they form tumors. Common properties: 1. genome structure and expression: Diploid gene with 2 single stranded RNA'a hydrogen bonded @ the end. The seq is LTR-gag-pro-pol-env-LTR-AAAAAAA. These 5 genes are common and important... a. LTR- includes the viral promoter and either R-pp-U3, or R-U5-PB-Leader. The viral promoter is self explanatory. R controls redundant sequences on the ends, U5 is essential to initiase RT. U3 is essential for integration and replication, The leader region has a signal for packaging of Genomic GNR into viral particles. PB is where the primer binds, PP is initiation for replication of strand 2. GAG- Includes the group specific antigens that distinguish retroviruses infecting one specis from another, and is translated and cleaved into 3-5 structural proteins including Matrix (MA), capsid (Ca), and nucleic acid binding protein. PRO- encodes the protease (cleaves GAG, POL, and some ENV. POL- Codes for RT and IN (integrase). Both essential for viral infection. The RT copies the genome, the IN integrates it into a host chromosome. ENV- Encodes type specific antigens distinguishing retroviruses of the same species. Plays a role in surface recognition of cell proteins and fusion of membranes during penetration ONC- Only in exogenous viruses- Responsible for their transforming ability. (different depending on strain, but always a cellular gene altered by becoming expressed as part of the provirus. Note again that the retroviral genome is the only one that is diploid, the only one that is synthesized and processed by the cell mRNA handling machinery, and it does not serve as mRNA early after infection. |
|
|
Non-Human Retrovirus Replication (RNA cancer virus)
|
Attachment: A reault of the interaction of the ENV coded SU Viral protein with any one of a wide variety of cell receptors.
Penetration: After binding, membrane fusion releases the core, mediated by clevage of the ENV protein releasing SU and allowing the TM (transmembrane?) portion to interact with the membrane. Reverse Transcription: Occurs within the core structure copying genomic RNA into cDNA. All retroviral genomes have a special tRNA bond to their 5' LTR PB region, serving as a primer. The result is an RNA/DNA hybrid. RT has RNASEH activity meanign that after making the hybrid it cleaves the RNA, and then transcribes the 2nd or + strand using the -cDNA strand as a template and the 3' LTR-PP sequence as a primer. Transport of Viral cDNA: The whole virion goes to the nucleus intact, and requires that the host undergo mitosis for a production infection (possibly it gets trapped by the dissolution and reformation of the nuclear envelope?) Integration: Linear DNA integrates at random sites forming the provirus, catalyzed by integrase (coupled breakage rejioning reaction inserting a complete copy of the viral genome). Synthesis of Viral RNA: Using cellular RNA polymerase II, is followed by translation into proteins. It is transcribed into a single RNA precursor, a portion of which remains intact and will be incorporated into new virus. The remainder gets spliced into 2 portions, one with GAG-PRO-POL, and the other ENV. Assembly and budding: GAG, PRO, and POL associate wtih the cell membrane and each other. Following assembly they are cleaved by viral proteases and the env product is inserted into the membrane via interaction with one of the GAG products. Release proceed by budding. |
|
|
Exogenous Retroviruses
|
Exogenous sarcoma/Aute Leukemia viruses induce tumors faster than endogenous in animals, as a result of their poession of transforming genes (V-Onc, Oncogenes). Nearly Identical genes (c-ONC, Protoconcogenes) are in non-infected human tumor cells. The genes are cellular in origen but were picked up by the virus and became transforming via point mutation, overexpression, inappropriately timed expression, expression in the wrong place, or production of fusion proteins via translocation of one gene next to antoher. Removal of these genes does not affect the viral potential to replicate. They are a diverse bunch of genes from a variety of hosts.
Oncogene Fxn: Many fall into 4 categories, each with important roles in regulation of normal cell proliferation... Growth factors Growth factor receptors Tyrosine Kinases/Signal transduction mediators Transcription factors |
|
|
Endogenous Retroviruses
|
They do not have transforming genes but cause tumor formation (although at a slow/inefficient rate). The location of viral genome insertion seems to be an important factor. The LTR is important here as it is capale of altering expression of genes. Thus if the provirusis integrated adjascent to a gene involved in growth regulation, you can cause transformation.
|
|
|
Human Retroviruses (General info)
|
They casue 2 diseases involving disturbances of the growth of one cell type (CD4 lymphocytes. It is central to the regulation of the immune system in general, and in this case it is either induced to proliferate (leukemia) by Human T-Lymphotropic virus type I and II, and is Killed by HTLV-III (LAV, ARV, HIV-I, HIV-II). Either result leads to immunosupression and severe opportunistic infections, AIDS, and unusual neoplasms (Kaposi Sarcoma). They have extreme trophism for CD4 marker cells as CD4 is the actual receptor. The classification of retroviruses is based on genetic, molecular, morphologial, and biological properties. Common properties:
1. All are exogenous retroviruses 2. All have tropism for human Helper T cells with CD4 antigen). 3. Infection leads to impariment of T cell fxn 4. Infection leads to syncytia/multinucleated giant ells in culture 5. Similar modes of transmission (sex, blood, birth) 6. Cross reactive p24 antigenic determinant 7. Probably of Africn origin 8. Will infect man as well as old world monkeys (multi species tropic) 9. Genome consists of a number of additional elements aside from the genes. Properties that distinguish them: 1. The ultimate fate of the infected cell. 2. differences in the primary genetic structure (different size and antigenicity) 3. Fxn of the trans-activating elements. 4. HIV has an elongated/triangular looking core. |
|
|
Human Immunodeficiency Viruses (HIV-I, HIV-II)- General Info, Epidemiology, Genome/Replication
|
A retrovirus AKA lymphadenopathy virus (LAV) and HTLVIII. A mamber of the lentavirus family infecting humans, monkeys and ungulates (like sheep in whom it causes dementia). It originated in chimps in africa and spread to man (zoonosis) in the mid 1900's). It is an enveloped + SS RNA with 2 copies (DIPLOID) dimerized with a tRNA-lys at the primer binding site. Capsid structure is irregular.
Epidemiology The initial epidemic started in the 80's as a cluster of Kaposi Sarcoma and PCP in MSM in San Francisco and NY. It lead to the discovery of HIV/AIDS, initial work on the virus, release of AZT, HAART therapy, and the revelation of chimps as the natural host. From MSM the virus quickly jumped to IV drug users, who donated blood frequently as a means of income, so from there to hemophiliacs. Genome/Replication Genomic Organization includes 9 unique genes flanked by the LTR's. The extreme genetic variation is the major vaccine obsticle and leads to resistance to HAART, as well as resistance to CTL's and phenotypic switching from CCR5 to CXCR4 trophism. It is the result of a lack of editing fxn ont he part of RT as well as RNA Pol II which transcribed the provirus, Strand switching (RT jumps strands in the middle of transcribing), and Superinfection/recombination. Today there are 9 different "clads" or strain of virus that are dramatically altered (Type C is common in Africa). Several of the genes are divided into non-contiguous pieces which are spliced together as an mRNA prior to translation. As the DNA can be read in 3 ways, as many as 3 genes can coexist on one DNA segment. GAG POL TAT (TAT-3, TA) REV (ART, TRS) VIF VPR VPU NEF The replication cycle is as with other human retroviruses from adsorption to integration, however due to tat, rev, and nef the expression of the provirus is different. Adsorption: Viral Gp120/Gp41 binds to CD4 with CCR5 (Macrophage trophic, initial infection) or CXC4 (T cell trophic) coreceptors. Penetration: Conformation change in Gp41 leads to release of its hydrophobic peptide initiating fusion. Reverse Transcriptase makes cDNA on the way to the nucleus, this is integrated as the provirus at a random site. Initial transcription is short until a full length slips out and is able to generate TAT protein which shuttles back in and increases transcription. Rev also goes back in to mediate early/late expression via altering nuclear export. Note that HIV-I synthesizes its proteins in the form of polyproteins that need to be processed by the HIV-I protease. It also can cause syncytia formation. |
|
|
Nosocomial Infection
|
As a house officer this will be the most common kind of infection that you will treat. e.g. There are 4x as many nosocomial infections as there are heart attack admissions. In about 3% of patients it contributes to the death of patients. Occationally there is an outbreak/epidemic of one kind of
Def: Infection occurs in an institutional setting (hostpial, nursing home, etc). This is different from iatrogenic infection which is physician produced from inadvertant or erroneous tx. Risk Factors: 1. Lots of tubes/lines etc...creating highways from the external to the internal environment. Ex- Endotrachial tubes, foley cath, surgery, wound drains, IV catheters. This bypass is a big immunocompromise. 2. Length of stay (longer is worse) 3. The sicker you are, the more likely you will get another infection. Furthermore the kind of original ilness and tx can dictate the kind of nosocomial infection (endotrachial tube --> airborn bacteria/fungi, Foley --> UTI etc) 4. Colonization (bugs live there but do not invade) with a bug increases the liklyhood that you can get an infection with that bacteria. Risk is generally highest in surgical patients and the rate is higher in large teaching hospitals as more invasve procedures and sicker people are there. What kind of infections do patients get?" 1. pneumonia (endotrachial tubes/tracheostamy) 2. Blood stream infections (IV) 3. UTI (Foley) 4. Wound infections (surgery/wounds) Most of these are environmental bacteria, aerobic, etc. Many are normal human flora that get into the wrong place. Control: 1. Hand Washing 2. Proper sterilization of equiptment and care of invasion sites. 3. Infection surveillance/staff training. 4. Antibiotics INCREASE risk of infection by eliminating normal flora |
|
|
HIV Genes
|
GAG- Late phase polyprotein precursor for core proteins MA (p17), CA (p24, basis for elisa/western), NC (p9/7 binds to RNA, Zinc fingers).
POL- ** early gene. Enzyme precursors PR (protease, cuts GAG into MA/CA/NC), IN (integrase), RT (reverse transcriptase) and RNAseH. RT DNA polymerase activity requires a primer and a template of either DNA or RNA. It requires MG or MN and lacks proofreeding. RNASEH activity is also encoded, but in a different area. ENV- Late phase polyprotein including gp120 (SU/Surface, viral receptor) and gp41 (TM/Transmembrane, mediates fusion and holds SU). Highly mutated domain. TAT- ** early gene. Transcriptional transactivator, increases expression of all 5' LTR linked genes, interacts wtih tar (transactivatioin response elements), required for replication. Before TAT, transcription terminate early, when 1 slips through, TAT makes transcriptional complexes competant**Gives HIV unique repliation character. REV- Regulator of virion protein expression. Promotes transition from early to late, activates transport of virion mRNA to the cytoplasm for translation, decreases early gene export. It recognizes specific sequences on ENV called the RRE (REV-responsive element), so ENV or full length exports occur. Essential for viral replication. **Gives HIV unique repliation character. NEF- Transcriptional silencer, accumulates very early and is not essential for viral replication (in fact disruption leads to higher replication levels, but decreased pathogenecity). Gets myristylated and locates to the plasma membrane where it downregulated expression of CD4 by enhancing endocytosis, and can activate/modulate CD4 T cell signaling pathways. May contribute to the establishment of latent infection. Precise fxn unknown. **Gives HIV unique repliation character. VIF- Viral infectivity gene accumulates late in infection and critical for ability to transmit infections to other cells. Not found in mature virus particle, but may be necessary for maturation as a protease. VIF defective virions do not produce full length DS DNA. Vif also inhibits cytidine deaminase in non-permissive cells, and leads to G to A genetic transitions. VPU: Viral protein U facilitates teh export of viral particles from the cell. Most remains cell associated when it is absent. VPR: Viral protein R is in the virus in multiple copies late in infection. It activates transcription of many cellular and viral promoters, and acts early during the "next" infection. |
|
|
UTI complicated vs. uncomplicated
|
Complicated: Structural or functional disease already present like stone, catheter, bladder
Uncomplicated: no known urinary problem, but may be elderly, diabetic, etc. |
|
|
UTI Risk Factors
|
Females are at greater risk in general
Host/immune factors: "Sticky Epithelium," mechanical incasion via catheter or obstruction. Also bladder dysfunction. Microbial factors: Colonization, Extracellular factors of the bacteria, resistant to immune system, and some form their own stones so they have a place to hide. Uncomplicated: Female sex Sexual activity Spermicide use Prior UTIs Some genetic factors: P blood type Non-secretor status Diabetes Complicated Pregnancy Advanced age Poor neurologic control of bladder Stones, tumor or other obstruction Catheter Diabetes Prior antibiotics |
|
|
UTI General Principals
|
The most common bacterial infection (nosocomial or otherwise) (1/3-1/2 of women will have one, women are 3x more likely to be hospitalized for UTI, esp in childbearing years)
It includes bacteria/fungi in the kdiney, renal pelvis, ureter or bladder. It usually causes urinary symptoms (but not always) and in men prostate involvement is common and challenging MIcrobiology: E. Coli is the majority (esp in ambulatory patients), but other gram -ves in hospital, especially pseudomonas aeruginosa. Some gram +'ves as well like staph saporophycitus, enterococci. Antibiotic resistance is also important. source of uropathogenic strains: Predominant fecal strains or in mixed flora, and sex partners share E. Coli strains (can predict upcoming UTI), and UTI caused by adherent strains has a greater risk of relapse. For some reason we are also seeing a spread of antibiotic resistant strains, but we do not know how they are spreading. Symptoms: Frequency, burning, urgency, pubic discomfort. Flank Pain (kidney) Fever, Chills, Constitutional features (may be the only symptoms in parapalegic patients...fever, malaise, etc). Some are asymptomatic, and we do not need to worry about it with tx not necessary with the exception of Pregnancy and post renal transplatns. For those patients we screen even asymptomatic patients. It is frequent with indwelling catheters or with advanced neurologic disease. Disease with similar symptoms: Vulvovaginitis with Trichomonas, Candida, and Bacterial vaginosis Urethritis, Sexually transmitted diseases, Gonorrhea, Chlamydia Prostatitis… Epididymitis UTI prevention Well documented: Avoid spermicides for contraception Avoid douching Cranberry juice (maybe, drink ALOT at least 2x per day, works like a P-type secreter!) Not helpful (but sometimes mentioned) Post-coital voiding (post sex, does not help!!!) Direction of wiping Increase fluid intake In healthy people- they are common, and tx outcomes are good. In sick folks, we see increased predisposition and variable outcomes. Recurrence is to be expected. We want to try to control mitigating host factors (diabetes etc..). For the sickest folks, signifiicant UTI's are common, and do not all need to be treated. We want to improve symptoms, and cure when possible. Recurrence is very common. |
|
|
E. Coli UTI
|
some serotypes are particularly likely.
Adhesins (various fimbriae) mediate sticking to the epithelium. They are not necessary for survival but are highly correlated with pathogenicity. All intestinal bacteria adhere to a degree, but a subset also have genital/urinary tract adhesion. This is generally less important in nosocomial infections (they get a free ride into the body in the hospital). P fimbriae attach to specific host sites (present on people with the P blood type, but not on people with the p blood type). Small p type then has a lower risk of UTI, as do P secretors (shed P into secretions decoying UTI bacteria) Other virulence factors include aerobactins, hemolysins, etc. Type 1 fimbriae is present in most strains of E. Coli and recognizes mannose containing receptors (stick to Tamm-Horsfall protein). Also the presence of lipopolysaccharide (Endotoxin) or Capsules (K-type) |
|
|
UTI DX and TX
|
DX
Physical findings- fever, flank tenderness, large bladder, suprapubic tenderness, neurologic symptoms. lab tests- Urinalaysis dipstick + for nitrites or leukocyte esterase test (1 + = likely, 2 + = Very likely). Miscroscopic exam of urine can help if we are not sure. We can culture but it will take 24 hours. and we will then need another 24 hours for susceptability and ID tests (culture is more confirmatory, and used to track resistance, and for pt with drug allergies/tx failure/hospitilization etc). Culture is + if there is > 10^4 bacteria (less may be contamination) and to check for monomicrobial vs. polymicrobials. Tx- Depends on clinical setting If no tx, symptoms are slow to resolve and there may be complication. Tx intensity should match the health of the pt (low for mild disease in healthy women, high for men or complex cases in women). Fluoroquinolones are oftenused. TMP/SMX is also good, but resistance is creepin up. Beta lactams are useful in pregnancy, but otherwise are 2nd line. Also Nitrofurantoin, Methenamine, and fosfomycin also have uses. Tx duration is variable, generally 3 days (men require longer courses to clear the prostate). Recurrent UTI's are 90% caused by new organisms, and are rarely associated with structural UT lesions, and respond to therapy. The patietns are generally healthy but "sticky." You can also give a script for antibioitcs to take for a while at first, and then intermittently after intercourse or with symptoms. If it comes back, see the doc (this works 1/3 of the time). Also keeping the bladder from overfilling, by some patients cathing themselves several times daily. THERE IS NO ANTIBIOTIC PROPHYLAXIS FOR COMPLICATED UTI/CATHETERIZATION |
|
|
Sexually Transmitted Diseases
|
US is the world leader in STD's (including adolescents who incorrectly think that oral sex is safer in terms of STD). Adults reentering the dating scene also leave their guard down.
Note that not all genital ulcers are STD's Note that a large %age of MSM with STD's have mixed infections. General Rule...when testing for STD's, always test for HIV as well. |
|
|
STD Herpes
|
HSV-I is the predominant virus causing the cold sore (20% HSV-II), not an STD generally but can be transmitted vir oral sex.
HSV-II IS ALMOST ALWAYS AN STD (EXAM). 1/4 of americans have STD, 1/5 have HSV-II, and >90% do not know they are infected. With PRIMARY genital herpes: Incubation time is 2-5 days. You get pustules which may rupture into an ulcer (or just to ulcer form on mucosal surfaces) and it may take 2-3 weeks to heal completely. MULTIPLE TENDER VESICLES IN DIFFERENT STAGES and systemic symptoms (malaise, fever, H/A, Myalgia) is characteristic of primary herpes. There is often associated lymphadenopathy (as with many STD's), the lymph nodes are firm, and tender. To urinate for women can be VERY painful. First episode is almost always the worst. Following primary infection Herpes takes refuge in the dorsal root ganglion. Type II is most likely to cause recurrence (I is less recurrence and less viral shedding, it is BETTER TO HAVE HSV-I THAN II IN THE GENITAL AREA). Recurrence can be triggered by Stress, Fever, UV/Sun, Other infections, Menses, Emotions, Diseases, Iatrotenic immunosupression, Dorsal Nerve Section, Trauma. RECURRENT HSV can be few to one lesion, with no systemic symptoms and little or no pain. DX: The most accurate way is via culture with a swab appropriate for viral transport, esp during a vesicle or open ulcer. Measure cytopathetic effect. Takes 24-48 hours. Also means that we can look for multinucleated cells (not as accurate, other herpes causes them), and EIA. 9/10 times a herpetic simplex process on the hand is misdiagnosed as a bacterial pustule. It happens mostly in healthcare workers as they touch oral herpes without gloves. Never touch any rash with no gloves. Tx Keep lesions clean, no antibacterials or steroids. Use analgesics, and perhaps oral antivirals like acyclovir (topicals are not great). Meds can also be used prophylacticaly. The vast majority (II) is TRANSMITTED BY PEOPLE WHO EITHER DO NOT KNOW THEY HAVE IT OR ARE ASYMPTOMATIC AT THE TIME (EXAM)!!!! Type I is only transmitted when you have lesions. |
|
|
STD Syphilis
|
Caused by Treponema Pallidum. The incidence spiked in the 90's as a result of cocaine/prostitution. There is a small upswing now, in gay/bisexual males.
Incubaton is between 10-90 days, is generally about 3 weeks. The characteristic lesion is a HARD chancre, painless, and there is lymphadenopathy but it is also non-tender. Not everyone gets a chancre, and as it is painless and dissappears in about 4 weeks, so many may not be trated. Then you get a rash (secondary syphilis) on the torso, PALM AND SOLE (like rocky mountain spotted fever). If they are still not treated, they can lead to tertiary syphilis with systemic badness. Dx via antibody tests and microscopy. NO RESISTANCE TO PCN, SO THAT IS WHAT WE USE (EXAM!!! like group A strep) |
|
|
STD Chancroid (haemophilus Ducreii)
|
In the US chancroid is rare among STD's. TROPICAL COUNTRIES ARE DIFFERENT, AS THE TOP STD THERE IS CHANCROID.
The size of the lesion is a major factor in transmission. Prostitution is the major risk factor. Typical lesion- Soft chancre (not firm or indurated), painful, pus-like base, associated painful large compressible Lymph nodes (bubos). If a pt touches one are then another, they can self innoculate. Dx Culture Tx wide variety. |
|
|
STD Granuloma Inguanali (not on exam, less than 100 cases in the us per year)
|
presents with donovan body, common in africa, indian subcontinent, etc.
Long incubation period. causes a serpigenous ulcer, large, spreading, painless. Does not culture well, scrape, crush, look for donivan bodies Pseudobubos |
|
|
STD Chlamydia
|
MOST COMMON REPORTABLE STD, MOST COMMON BACTERIAL STD, OR STD IN ALL (exam).
Can casue LGV lympho granulomavenerium (ulcerative) esp in MSM and elswehere. There are actually many serotypes, but it is trachomatis that we are most concerned about. The majority of people with LGV have a small, painless, fleeting ulcer. The ENORMOUS tender groin lymphnodes (compressible-bubos) are diagnostc. The inguinal ligament is outlined (a GROOVE SIGN [EXAM]) which can be aspirated for defnintive dx. Also, the presence of inclusion bodies. It can spread to the space between the liver and diaphragm causing perihepatitis. Transmission: GONORRHEA AND CHLAMYDIA CAN BE MILD/ASYMPTOMATIC IN WOMEN LEADING TO THE SPREAD OF INFECTION (EXAM). Testing should be done (yearly?) in women who meet 2 of the following criteria: 1. Under 25 and sexually active. 2. Having a new sex parther in the last 2 months 3. Not consistently using barrier contraception 4. Mucopurulent cervical discharge 5. Cervical bleeding induced by gentle swabbing. Also testing is indicated during pregnancy to prevent neonatal chlamydia which is BAD. The scarring of the lymphnodes can be so bad it can lead to elephantiasis of the genitals (esthiomene) In fact CHLAMYDIA CAN CASUE A WIDE RANGE OF DISEASE AND CONDITIONS (EXAM). Tx: Erythromyin, doxycycline, Z-pak. |
|
|
Summary of STD Etiology
|
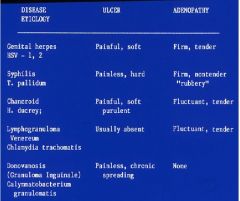
Remember, do not know Donovanosis for exam.
|
|
|
STD Gonorrhea
|
Gram - diplococci.
Clinical Disease: Incubates 2-10 days with sudden onset, common and sometimes severe dysuria, and large amount of purulent discharge. Similar to chlamydia. GONORRHEA AND CLLAMYDIA CAN BE MILD/ASYMPTOMATIC IN WOMEN LEADING TO THE SPREAD OF INFECTION (EXAM). Tx- DO NOT USE quinolones, we use advanced cephalosporins now. When we Tx we need to remember that about 50 % of patients will have another bug that can cause urethritis, esp chlamydia. |
|
|
Causes of Pelvic Inflamatory Disease
|
It can be caused by any of a number of bugs...
Gonorrhea, Bacteria, Chlamydia, and unknown/mixed causes. |
|
|
Fauci Graph
|

Fauci Graph
|
|
|
Clinical features of Primary HIV-I infection
|
General:
Fever Anorexia Malaise Myalgia lymphadenopathy pharentigis Neurological: Retro-orbital pain headache meningitis enephalitis peripheral neuropathy Guillain-barre like syndrome GI: Abdominal pain N/V Diarrhea hepatitis GI Hemorrhage Dermatological: Erythematous macular or maculopapular rash urogenital ulcerations |
|
|
Human Immunodeficiency Virus (HIV-I, HIV-II), Transmission, Pathology, Dx, Tx
|
Transmission:
In spite of the increased incidence with anal intercourse (the colon has a huge receptive zone for the virus, almost all of the transmission in Africa and globally is heterosexual. In the US it is still third behind homosexual and IVDR here. Hemophiliacs are now safe as factors are made via recombinant technology, and screenign techniques are preventing use of infected transfusion products. Neonates can be infected in utero (swallow amniotic fluid), peripartum (reduced via C-section [result of M vs T?]), and through breast feeding. The former 2 can be almost eliminated using meds, and the last by Mom, so we have a shot here, but even if the baby is not infected, the likly loss of the mom early in babies life increases mortality. Infection is also helped as the baby has Mom's immunity which HIV has already learned to bypass. Pathology See Faci Graph for progression and detailed symptomatology. Initial infection is often in monocytes via CCR5 trophism in the GI or UG tracts. It eventually moves to CXCR4 trophism and infects CD4 T cells forming a reservior and causing the immunosupression that kills the pt. Primay infection often is associated with flu/IM like symptoms, and leads to a period of clinical latency in which the virus may still replicate. Reactivation/secondary infection and cancer result from loss of cell immunity Common associated neoplasms include KS (from KSAHHV), Lymphoma and hodgkins disease (from EBV), Cervical carcinoma (from HPV, should be reduced via the HPV vaccine), and other garden variety tumors. The Chronic inflamtion can lead to HIV Associated wasting (loss of white fat), and lipodistrophy (HIV and HAART associated, as white fat goes down, brown fat goes up and deposits creating buffalo hump). End stage AIDS often includes dementia as toxic substances are released by immune cells, infected cell, and the viral proteins themselves are toxic. Dx: Oral hairy Leokoplakia (From EBV) is often among the first signs. ELISA for p24 antibodies is the Gold Standard. It is sensitive (false +), as is Western for antibody to other HIV antigens. CD4 count and HIV-I RNA load are also markers to dictate/monitor therapy. We need to analyze a patients strain to determine susceptaility to HAART, specificaly we genotype the POL gene. We should also consider the patients genetics, and readiness for therapy in selecting a regimen. Tx: Nucleoside Inhibitors of RT NON-nucleoside inhibitors of RT Protease Inhibitors HAART Entry Inhibitors Integrase Inhibitors (experimental) Induction therapy with IL-2 and AZT to eliminate latent reserveriors. Vaccines are currently experimental (but includes DNA vaccines followed (transmitted?) by viral vectors). |
|
|
Define HIV infection, AIDS, Opportunistic infection
|
HIV infection
Any patient infected with the human immunodeficiency virus. Note that the virus makes 10 billion particles daily, and we turn over 1 million cd4 cells daily, but the CD4's get depleted over time crippling the immune system. AIDS Any person with HIV that has a CD4 count less than 200 or has had an opportunistic infection Opportunistic infection An infection caused by an organism that would otherwise not be a pathogen (Pneumocystis pneumonia, toxoplasmosis, etc) |
|
|
Monitoring of HIV Patients and initiation of therapy
|
The CD4 cell count-
in general, normal is above 500. When the CD4 gets below 200, the patient is at risk for opportunistic infections The HIV viral load- in general measures the amount of replication of the virus in the body. The lowest level of detection is 50 copies, avg for an HIV+ patient is 75,000 Therapy is indicated when: When the CD4 < 350 OR When the viral load is > 100,000 copies OR When the patient is symptomatic (weight loss, fever, anorexia, etc) AND The patient is ready for therapy (trumps everything, is he on drugs, homeless, etc.). This is becaue the pt must not miss any doses (missing 1/20 is enough to make therapy fail). The pt must call with any issues at all, and should not stop without telling the Doc (True of all his meds). Always start with 3 or more drugs, generally 2 NRTI's + 1 PI/NNRTI.... Most often either AZT/3TC OR TDF/3TC (can substitute FTC in 3TC’s place) With- Efavirenz Lopinavir/Ritonavir (kaletra) Fosamprenavir + Ritonavir Atazanavir + Ritonavir |
|
|
Nucleoside Reverse Transcriptase Inhibitors (NRTI's)
|
Commonly Used. Most have a generic name, abbreviation, and brand name.
Side effects include headache, nausea, diarrhea, darkened finger nails, anemia, and increased creatine kinase. Some also may cause hypersensitivity and if so must be discontinued. Zidovudine, AZT, Retrovir® Lamivudine, 3TC, Epivir® Abacavir, ABC, Ziagen® Emtricitabine, FTC, Emtriva® |
|
|
Nucleotide Reverse Transcriptase Inhibitors (NtRTI's)
|
class side effects include:
Lactic Acidosis: Stavudine and didanosine are most associated when in combination But may be seen with other NRTI as well A result of the inactivating effects on mitochondrial DNA replication Peripheral fat wasting: Stavudine most associated Both are believed to be secondary to mitochondrial toxicity Some reports of fanconi Syndrome with Tenofovir (Viread [renal failure and proximal tubular damage]) |
|
|
Fixed Combinations
|
There are some meds which combine NRTI's and NtRTI's including:
Combivir® (fixed tablet of lamivudine 150mg and zidovudine 300mg) given bid Trizivir® (fixed tablet of lamivudine 150mg, zidovudine 300mg, and abacavir 300mg) given bid Epzicom® (fixed tablet of lamivudine 300mg and abacavir 600mg) given once daily Truvada® (fixed tablet of emtricitabine 200mg and tenofovir 300mg) given once daily Combinations of PI's: Kaletra- Lopinavir/Ritonavir..side effects includ nausea, diarrhea, pancreatitis |
|
|
Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI)
|
Side Effects includ CNS Abnormalities, Nightmares, anxiety, sleepiness, hepatotoxicity and rash. Efavirenz is the only one that cannot go to pregnant women as it may cuase neural tube defects. Hepatotoxicity with Nevirapine is due to pretherapy CD4 count so it is not recommended for women >250 or men >400 (Although if the CD4 climbs after therapy has startd it is ok.
Nevirapine, Viramune® Efavirenz, Sustiva® |
|
|
Protease Inhibitors
|
Commonly Used. Side effects inlcude:
Extremely potent cytochrome p-450 inhibitors Elevations of triglycerides Elevations of cholesterol Elevations of glucose Central adiposity Nausea, Vomiting, Diarrhea, rash. Note that they are all risk factors for Heart disease, and there are some associated cases, but they are not as predictive as classic risk factors. Fosamprenavir, Lexiva® -has sulfa Atazanavir, Reyataz®- inhibits glucuronidation (jaundice) Ritonavir, Norvir®- not tolerated as antiviral, but inhibits P-450, so used as a booster for other meds. Tipranavir, Aptivus®- Also causes intracranial bleeding. Used only with ritonavir. Darunavir, Prezista®- can only be taken with ritonavir |
|
|
Fusion Inhibitors
|
Note that the virus first binds CD4, and then a coreceptor (CXCR4 or CCR5) causing a conformational change that alters GP41 confirmation making it into a drill that fuses the 2 membranes.. These inhibit the bending/folding back of GP41.
T-20, enfuvirtide, Fuzeon®- Sub-Q injection, mixed and shot by pt BID. |
|
|
Single Dose Regimen
|
Atripla® was approved in August, 2006
It is a combination of tenofovir/emtricitabine and efavirenz It is one pill once daily, the first complete regimen in one pill |
|
|
New Classes
|
CCR5 inhibitors- require trophism typing, a $2000.00 test.
Integrase Inhibitors- irreversably binds integrase before it integrates the DNA. |
|
|
Sepsis
|
Definition: Sepsis (AKA Systemic Inflammatory Response Syndrome (SIRS) is the host response to an invading microorganism, usually manifested by at least two of the following-
Fever/Hypothermia Tachycardia Tachypnea Leukocytosis or Leukopenia Note that SIRS can be the result of many things including lung aspiration. Sepsis is SIRS as a result of infection. Severe Sepsis/Sepsis Syndrome- Sepsis with organ dysfunction distant form the site of infection, hypoperfusion, or hypotension. Septic Shock- Severe sepsis requiring pressor therapy despite adequate hydration. It is generally a disease of the very old and very young. Gram -'ve used to be the #1 cause, but gram + are now in the mix, including Staph A and Sap, as well as Enterococi and Candida. Most of the time it is good old pneumococcal pneumonia. It is the result of microbial invasion --> host recognition of invasion --> host response --> organ dysfunction. Note that the infection can lead to bacteremia, or can be localized excreting toxins. Mostly there is no prognositc difference, with the exception of Menningococcus. Cause: endotoxin (LPS) on the cell membrane fo gram - bacteria. The toxin is released as the bacteria are killed. That endotoxin is recognizedby the TOLL-Like receptor on fibroblasts and macrophages. |
|
|
Regocnition and Response to infection
|
Recognition: The microbes establish the infecton, but their highly conserved molecules (LPS) are recognized by dendtiric cells and monocytes. A complex of LPS/Endotoxin Bindin Protein/Toll/CD14 trigger intracellular signaling leading to a host response.
Host Response: Alteration in transcription and secretion of inflammatory cytokines TNF, Interleukins, NO. Their presence attracts neutrophils (PMN's), activates macrophages, and stimuates TH1 cells. Appropriate results include: Fever Tachycardia Leukocytosis Peripheral vasoconstriction Procoagulant state Local pro-inflammatory factors Systemic anti-inflammatory factors preservation of euglycemia (as we will often be naorexic). |
|
|
Proper Infection Response Effects
|
Fever-
Consider not treating low feves immediately in-house.. May inhibit bacterial growth Increase bactericidal activity of neutrophils and macrophages Peripheral vasoconstriction may divert increased blood flow to infected area Local Nitric Oxide (NO) allows for vasodilation Tachycardia- Increase cardiac output Increase blood flow to injured tissue, to deliver all kinds of goodies, white cells, etc. Leukocytosis- Mobilizes neutrophils into the circulation and prevention of neutrophil adhesion to non-inflamed endothelium (the normal state) via: Glucocorticoids Epinephrine We demarginate systemically and attract them locally. Local cytokines (TNF, IL-1) attract neutrophils Peripheral vasoconstriction- Divert blood supply to vital organs and to infected/injured site Procoagulant state- Primitive immune response Wall off infection Increase fibrinogen synthesis Decrease synthesis of protein C and anti-thrombin III Systemic anti-inflammatory factors- Cytokine antagonists, anti-inflammatory interleukins, ACTH and cortisol (stim release of steroids), epinephrine Maintain Euglycemia- Epinephrine, cortisol, glucagon, cytokines Local pro-inflammatory factors- TNF, IL-1, Nitric Oxide, IFN-gamma |
|
|
Organ Dysfunctin in Sepsis
|
Nervous System-
Cognitive Deficits Confusion, esp. in the elderly Hypothalamic-Pituitary-Adrenal Axis Loss of normal ACTH release Possible glucocorticoid insufficiency Loss of vasopressin release- Depletion of vasopressin in posterior pituitary Loss of baroreflex Hypotension Cardiovascular- Myocardial “Stunning”- Decreased contractility and ejection fraction Hypovolemic shock(Vasoconstrictive) Vasodilatory (Warm) shock Loss of systemic vasoconstriction (low BP, warm fingers and toes...not good) Downregulation of adrenergic receptors Microcirculatory dysfunction Poor organ perfusion Lactate production (excerbates acidosis) Pulmonary: Initial hyperventilation & respiratory alkalosis Acute lung injury Alveolar epithelial injury Increased Aa gradient Bilateral infiltrates on X-Ray Systemic inflamation leads to Adult Respiratory Distress sydrome (ARDS) Increased work of breathing Right to left shunting Increased dead space Mechanical ventilation not uncommon GI Ileus is common (lack of peristalsis) Gastric epithelial metabolic decrease/Cellular Apoptosis Hepatic insufficiency Increased transaminases, bilirubin, Alkaline Phosphatase Usually not severe Renal May become oliguric (low output) Decreased GFR & increased creatinine Usually reversible once sepsis resolves Endocrine Adrenal Insufficiency Adrenal hemorrhage Inadequate reserve Hyperglycemia Tissue insensitivity to glucose/insulin resistance Coagulation Disseminated Intravascular Coagulopathy Small vessel thrombosis (rare) Endothelial dysfunction Thrombocytopenia Immune System Prolonged sepsis leads to an immunocompromised state (apoptosis) Decreased macrophage responsiveness to cytokines Loss of MHC class II molecules T-helper Lymphocyte transition from Th1 predominance (IFN-gamma, pro-inflammatory) to Th2 (IL-10, anti-inflammatory) Loss of tight regulation, switch to local antiinflmatory and systemic infalmation. B & T cell apoptosis |
|
|
Sepsis Therapy
|
Broad Spectrum Antibiotics
Gram positive, gram negative, and consider Candida Surgical Drainage if warranted Fluids, blood products, pressors Activated protein C? Insulin Others Some things that have/do fail: Activated Protein C (in less severe sepsis) Endotoxin antibodies High dose corticosteroids (Possible role of low dose steroids) Insulin resistance? Granulocyte colony stimulating factor (GCSF)- No difference even when neutrophil count was 70,000 Prophylactic IFN-gamma, a pro-inflammatory Th1 cytokine, was unable to prevent severe sepsis in surgical and major trauma patients |
|
|
Sepsis Therapy: Activated Protein C (Drotrecogin Alfa), Insulin
|
Activated Protein C (anticoagulant)
Inflammation/anti-inflammation major component of sepsis Coagulation pathway intertwined with inflammatory pathway First severe sepsis agent proven to work Four day infusion of human recombinant activated Protein C Multiple exclusion criteria Activated protein C may be anti-apoptotic agent, and therefore pro-inflammatory Insulin: Patients with bacteremia and tight glucose control (80-100mg/dL) had lower mortality than those treated with standard therapy (180-200mg/dL) 12.5% mortality vs. 29.5% |
|
|
Acute Inflamation
|
Causes: Bacterial Infections (with few exceptions)- Leads to necrosis, abscess, and scarring, with the bacteria often ID's histologically (Strep Pneumonia often resolves with out this tissue dmg).
Some Fungal Infection, may also produce infarction via vasotropism. ID'd in tissue. Parasitic Infections. manifestations: altered blood flow Presence of neutrophils Results: Resolution Progression to chronic inflamation and repair. (chronic may follow acute or come alone) |
|
|
Chronic Inflamation
|
Cause: Some Fungal Infection, may also produce infarction via vasotropism. ID'd in tissue.
Parasitic Infections. Viral Infections causing also necrosis inflamation, cytopathic effects, cytoproliferative effects and inclusions. Manifestation: Presence of PMN's Result: May persist or dissappear and may result in tissue distruction or repair. |
|
|
Granulomatous inflamation
|
Cause: Granulomatous inflamation is a rxn to indigestable material. Mycobacterium Infection (also caseous necrosis with Gran Inf) which is difficult to ID histologically (req. culture).
Some Fungal Infection, may also produce infarction via vasotropism (crypto can be granulomatous can be present with no necrosis/inflamation in many tissues). ID'd in tissue. manifestation: Presence of granulomas - localization of activated macrophages. Multinucleated giant cells may be present (not defining either way, but a good clue that you are looking @ a granuloma) Results in repair |
|
|
Repair
|
Proliferaiton of fibroblasts and endothelium (forms granulation tissue)
As it evolves, the cells regress and tissue presence increases becoming a fibrous scar. Can result in Gliaosis in the NS and loss of fsn of the repair tissue compared with the original. |
|
|
Changes in the Infectious Disease patterns
|
New diseases
New geographic range for old disease (travel) New populations at risk (we implant foreign bodies etc...) New tricks for old bugs (group A strep can now cause toxic shock syndrome) New faces for old infections (infant botulism with C. Botulism living in them rather than ingesting toxin) New modes of transmission (organ txplant) New niches for old bugs (Pseudomonas in sneakers-due to puncture of foot, causes osteomyelitis) |
|
|
Causes of chages in infectious disease patterns
|
Changes in lifestyle:
travel patterns, sexual behavior, pets, dietary trends (sushi/gefilte fish-Diphlbothrium (fish tape worm)), E. Coli in sprouts, Snow peas with cycospora, Cholera in seaweed and crabs, working parents (MANY outbreaks at daycare centers), leisure activities (Pseudomonas folliculitis/legionairs/amoebic keratitis[contacts] and otitis in hot tubs, salmonella in marijuana which decr. gastric acid). (E.G. MSM levels of gonorrhea decreased as they were educated re: HIV and they changed. Also Sex as a profession led to HIV spreading.) Impact of immigration. Impact of medical progress (Many more people live to old age and many outbreaks occur in nursing home settings to pt's already febile). vacines for MMR, HIB, etc. but opportunistic infections in txplants and foreign body implant *Staph Epidermiditis*, Dura Mater graphs causing CJD, rabies Infected donor's) Impact of weather (Tsunami survivors got pulmonary melioidosis [not endemic], all 6 had near drowning aspirations, Vibrio in Katrina survivors) Impact of international politics. (rabies increase in china in '03 with increase of pet dogs, rarely vaccinated. Religion- Botulism from peyote in native americans. |
|
|
Smallpox- features that favored eradication
|
No subclinical/asymptomatic cases
Contagiousness accompanies rash (not days before) Only get it once No animal reservoir No chronic carrier state Cheap, stable, effective, easy to give vaccine |
|
|
Alternative Medicine
|
40% of people going to MD’s are doing something as well…many AIDS pts. They may not volunteer it, but if you ask they will tell you. MVI, various tx.
AIDS- many people take capsules containing rattlesnake meat powder, often contaminated wtih salmonells Arizonae. Women developing tetanus after IM injection of fetal sheep cells (alternative for Rhumatism), non previously immunized, many died. Legionaires disease from contaminated water in babies delivered under water. |
|
|
Epidemiology
|
The most HIV is present in Sub-saharan Africa (1st) and SE Asia (2nd). The fastest growth of HIV infection is in eastern Europe and SE Asia. Throughout the world heterosexual contact is the most common mode of infection, and HIV is the 2nd most common killer behind TB. In the US originally whites were the most infected, but blacks have overtaken them, a real disproportionately large %age of cases are in blacks, esp in women. Other minorities also made up a disproportionately large %age (hispanics). In the US MSM still has the highest %age of cases, however high risk heterosexual contact is second and growing (*different than the world at large), again esp in black females. In the world, >95% of new infections are in low-mid income countries, >50% in women. Many with no meds/labs/docs/infrastructure etc.
In the Us we are seeing the number of new cases level, but the prevalence is increasing. That is because the number of deaths is decreasing, so more living people have the disease. Note that the death rate is higher among black men than women. We saw a major shift in the mid 90's due to the introduction of the first protease inhibitors and combination therpy protocols. |
|
|
Fauci Diagram II (insert diagram)
|
The squares are basically viral load… the drop is due to CD4 activation/replication which causes increased viral replication as they inhabit CD4’s, killing the CD4 cells. The fast paced drop in cd4/increase in viral load is not well explained….some theories, no proof. Note that T cells as well as monocytes have CCR5, so that it can actually get into both monocytes and T cells on day 1 depending on the strain.
|
|
|
Acute Retroviral Syndrome
|
Days to weeks after HIV infection a pt will present with a classic Mono-Like syndrome, often accompanied by Thrush (*more likely with HIV)
Most common symptoms are: Fever Fatigue Rash (maculopapular) Lymphadenopathy Pharyngitis Thrush Lymphopenia (low lymphocyte count) Thrombocytopenia (low platelets) Normal or slightly low CD4 count **Note that at this point the HIV ELISA may likely be negative, so we make the diagnosis bases on the P24 antigen test and HIV-RNA viral load (usually high (>300K). At this point the likleyhood of developing AIDS within 3 years is based on a combination of your CD4 count and Viral Load. |
|
|
AIDS Opportunistic Infections
|
Opportunistic infections in general are those that are not normally infectious (in a person with a normal immune system) as well as thsoe that occur frequently assocaited with the condition.
With HIV we can predict the liklihood based on CD4 Count (note that for each lower count, all of the diseases above the level are also possibilities) <200 Pneumocystis jiroveci Pneumonia <100 Cryptococcal meningitis Toxoplasmosis <75 Cytomegalovirus Mycobacterium avium complex Some others are not predictable using CD4, and may occur anytime: Tuberculosis Mucocutaneous candidiasis Hepatitis C Bacillary angiomatosis Progressive multifocal leuko-encephalopathy HIV dementia Malignancies |
|
|
Epidemiology
|
The most HIV is present in Sub-saharan Africa (1st) and SE Asia (2nd). The fastest growth of HIV infection is in eastern Europe and SE Asia. Throughout the world heterosexual contact is the most common mode of infection, and HIV is the 2nd most common killer behind TB. In the US originally whites were the most infected, but blacks have overtaken them, a real disproportionately large %age of cases are in blacks, esp in women. Other minorities also made up a disproportionately large %age (hispanics). In the US MSM still has the highest %age of cases, however high risk heterosexual contact is second and growing (*different than the world at large), again esp in black females. In the world, >95% of new infections are in low-mid income countries, >50% in women. Many with no meds/labs/docs/infrastructure etc.
In the Us we are seeing the number of new cases level, but the prevalence is increasing. That is because the number of deaths is decreasing, so more living people have the disease. Note that the death rate is higher among black men than women. We saw a major shift in the mid 90's due to the introduction of the first protease inhibitors and combination therpy protocols. |
|
|
Fauci Diagram II (insert diagram)
|
The squares are basically viral load… the drop is due to CD4 activation/replication which causes increased viral replication as they inhabit CD4’s, killing the CD4 cells. The fast paced drop in cd4/increase in viral load is not well explained….some theories, no proof. Note that T cells as well as monocytes have CCR5, so that it can actually get into both monocytes and T cells on day 1 depending on the strain.
|
|
|
Acute Retroviral Syndrome
|
Days to weeks after HIV infection a pt will present with a classic Mono-Like syndrome, often accompanied by Thrush (*more likely with HIV)
Most common symptoms are: Fever Fatigue Rash (maculopapular) Lymphadenopathy Pharyngitis Thrush Lymphopenia (low lymphocyte count) Thrombocytopenia (low platelets) Normal or slightly low CD4 count **Note that at this point the HIV ELISA may likely be negative, so we make the diagnosis bases on the P24 antigen test and HIV-RNA viral load (usually high (>300K). At this point the likleyhood of developing AIDS within 3 years is based on a combination of your CD4 count and Viral Load. |
|
|
AIDS Opportunistic Infections
|
Opportunistic infections in general are those that are not normally infectious (in a person with a normal immune system) as well as thsoe that occur frequently assocaited with the condition.
With HIV we can predict the liklihood based on CD4 Count (note that for each lower count, all of the diseases above the level are also possibilities) <200 Pneumocystis jiroveci Pneumonia <100 Cryptococcal meningitis Toxoplasmosis <75 Cytomegalovirus Mycobacterium avium complex Some others are not predictable using CD4, and may occur anytime: Tuberculosis Mucocutaneous candidiasis Hepatitis C Bacillary angiomatosis Progressive multifocal leuko-encephalopathy HIV dementia Malignancies |
|
|
Epidemiology
|
The most HIV is present in Sub-saharan Africa (1st) and SE Asia (2nd). The fastest growth of HIV infection is in eastern Europe and SE Asia. Throughout the world heterosexual contact is the most common mode of infection, and HIV is the 2nd most common killer behind TB. In the US originally whites were the most infected, but blacks have overtaken them, a real disproportionately large %age of cases are in blacks, esp in women. Other minorities also made up a disproportionately large %age (hispanics). In the US MSM still has the highest %age of cases, however high risk heterosexual contact is second and growing (*different than the world at large), again esp in black females. In the world, >95% of new infections are in low-mid income countries, >50% in women. Many with no meds/labs/docs/infrastructure etc.
In the Us we are seeing the number of new cases level, but the prevalence is increasing. That is because the number of deaths is decreasing, so more living people have the disease. Note that the death rate is higher among black men than women. We saw a major shift in the mid 90's due to the introduction of the first protease inhibitors and combination therpy protocols. |
|
|
Fauci Diagram II (insert diagram)
|
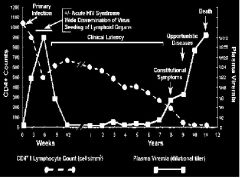
The squares are basically viral load… the drop is due to CD4 activation/replication which causes increased viral replication as they inhabit CD4’s, killing the CD4 cells. The fast paced drop in cd4/increase in viral load is not well explained….some theories, no proof. Note that T cells as well as monocytes have CCR5, so that it can actually get into both monocytes and T cells on day 1 depending on the strain.
|
|
|
Acute Retroviral Syndrome
|
Days to weeks after HIV infection a pt will present with a classic Mono-Like syndrome, often accompanied by Thrush (*more likely with HIV)
Most common symptoms are: Fever Fatigue Rash (maculopapular) Lymphadenopathy Pharyngitis Thrush Lymphopenia (low lymphocyte count) Thrombocytopenia (low platelets) Normal or slightly low CD4 count **Note that at this point the HIV ELISA may likely be negative, so we make the diagnosis bases on the P24 antigen test and HIV-RNA viral load (usually high (>300K). At this point the likleyhood of developing AIDS within 3 years is based on a combination of your CD4 count and Viral Load. |
|
|
AIDS Opportunistic Infections
|
Opportunistic infections in general are those that are not normally infectious (in a person with a normal immune system) as well as thsoe that occur frequently assocaited with the condition.
With HIV we can predict the liklihood based on CD4 Count (note that for each lower count, all of the diseases above the level are also possibilities) <200 Pneumocystis jiroveci Pneumonia ** <100 Cryptococcal meningitis Toxoplasmosis ** <75 Cytomegalovirus ** Mycobacterium avium complex ** = Prophylaxis Recommended @ CD4 level Some others are not predictable using CD4, and may occur anytime: Tuberculosis Mucocutaneous candidiasis Hepatitis C Bacillary angiomatosis Progressive multifocal leuko-encephalopathy HIV dementia Malignancies |
|
|
Self Limiting URI (children)
|
Epidemiology/Risks
Mostly kids under 2 have 5-10 symptomatic episodes/year, more than 10 if in daycare, many more asymptomatic than this. Decreases with age Clinical: Fever, rhinorrhea, nasal congestion, hoarseness, croupy cough, conjunctival infection, rash Diagnostic: Clinical, exclusionary DX. Preserved sense of well being, continuing milstone practice etc.. Causes/Tx Rhino, parainfluenza, RSV, adenovirus, coronavirus, metapneumo, influenza Enterovirus in the summer |
|
|
Acute Otitis Media (children)
|
Epidemiology/Risks
More common under 2 years (due to small size of eustcian tubes) parents smoking is a big risk, other environmental irritation, URI. Over 2, Pneumococcus Clinical Mostly fever, Otalgia (they pull/push/rub ear, act like it hurts) hearing bad, might hear the tube opening and closing. Rarely supputive (pus comes out when tympanic membrane ruptures) Generally fussy Diagnostic: Pneumatic Otoscopy (look in and use the bulb syringe to inflate it and see if the moves. Does not hurt) Membrane is erythamatous, opaque, bulging, immobile (see above). Need immobile + something else for Dx. (many viruses have all of the others, but the membrane moves) Causses/Tx: Pneumococcus Hemophilus Maraxal cataralis Tx Amoxacillin (90mg/kg/dy). If still bad after 3 days, give amox + clavulanate [augmentin] (to inhibit B lactamases, like in some hemophilus and maraxal, ) 70% self limited, but less than 40% if it is pneumococcus, so if the kid has all the findings, 90% give antibiotic. Always Tx kids under 2. |
|
|
Acute Sinusitis (children)
|
Epidemiology/Risks:
Any Age, Had a URI with persistant cough for 10 days, or has fever at the end of the cough. Clinical: Fever (often), facial pain, Teeth Hurt (maxillary sinus), periorbital edema, frequently pirulent rhinorhea (green stuff coming out of the nose). Not all need be present for Dx. Diagnostic: Clinical Causses/Tx: Pneumococcus Hemophilus Maraxal cataralis Tx Amoxacillin (90mg/kg/dy), high dose. |
|
|
Childhood Pneumonia (general)
|
Symptoms:
Fever, Breathing (rapid, difficulty, graveling), cough, dereased feeding, vomiting (cough, tough breathing..), Abd pain (esp bacterial on R lower lobe bacterial pneumonia), Neck pain (upper lobe pneumonia), chest pain Signs: Watch breathing for tachypnea (rate faster than normal), Audible wheezing, working --> retraction (intercostals [1st], subcostal [2nd], supersternal [3rd]), nasal flairing (worse than retracting), listen if possible, Fever level (rectal). –'ve abd exam x/c splinting of chest/pleuritic CP. Lower lung abnormalities Rales/crackles (end inspiratory, Tubular sounds, decreased sounds, Egophany on crying, rarely pleural friction rub (LOUD crackle on inspiration) Bacterial vs Viral vs Mycoplasma |
|
|
Bacterial Childhood Pneumonia
|
Age
Any, More common <2yo(less common with pneumococcal vaccine) Temp Any, >39 (103+) Onset Abrupt (fever resp dist), follows virus, Fever/ Others ill no Assoc symptoms Generally ill, bacteria are too specifically trophic. Cough Wet (will not show sputum) Pleuritic chest pain **Generally no, but if they do, they splint…If it is present it is definite bacterial (or PE) *degree of illness resp distress, resp rate lethargy, lack of self interest/environment, etc…toxicity; is much worse than physical exam would indicate (looks awful, we don’t hear much) Physical Exam Alveolar/peripheral disease, anatomically confined. ***Rales, wheezes, tubular breath sounds, etc are anatomically confined by the airways/lobe and ant/post X-ray Anatomically confined abnormalitiy/consolidation WBC (pneumococcal-neutrophilia) >15,000 many neurtophils Sputum If intubated we get it and look for neutrophils/pirulencs Pleural fluid Maybe Treatment Ab (pneumococcus, maybe Str A, rarely staph (pleural fluid), Amoxcillin high dose |
|
|
Viral Childhood Pneumonia
|
Age
Any, More common <5, but really any Temp Any, <39 (no viremia) Onset ** Gradual (both started with URI (nonspecific rhinorrea/congestion) Others ill ** Yes, always, Nonspecific URI/Conjunctivitis, rash, croupi cough, hoarseness**, Sore throat, myalgia, this or last week (concurrent b/c resp vir have short incu time) **the more mucus membranes involved, the more likely to be a virus** Assoc symptoms Nonspecific URI/Conjunctivitis, rash, croupi cough, hoarseness**, Sore throat, myalgia, this or last week (concurrent b/c resp virus have short incu time) Cough Dry/non-productive Pleuritic chest pain No *Degree of Ilness proportional to the findings (looks ok, hear b/l rales, wheezes, tachyp etc.) Physical Exam Rales, wheezes (cause upper airway inflammation), Diffuse, B/l (not anatomically confined) X-ray b/l diffuse interstitial disease WBC <15,000 many mononuclear cells Sputum No predominant organism, lymphocytes Pleural fluid No Treatment RSV, Parainflueza, supportive care, not antivirals generally |
|
|
Mycoplasma Childhood Pneumonia
|
Age
School age – young adults Temp Usually <39 Onset 2 phases, abrupt onset fever, myalgia, sore throat, flu (fades) **Gradual increase in cough (disease characterized as slow onset) Others ill Frequently, flu like, hx is over weeks (not concurrent) Assoc symptoms (increased with smoke in environment/mucus membrane irritation) Cough Hacking, paroxysmal (there and gone), productive Pleuritic chest pain No *degree of Illness Nothing…walking/atypical pneumonia. Hear rales, pt looks ok. Physical Exam Anatomically Confined findings, sometimes a single lobe or contiguous lobes. X-ray Patchy anatomically confined WBC Not helpful, 60/40 poly’s/mono… Sputum May have lymphocytes or poly’s Pleural fluid No Treatment If under the age of front teeth eruption azithromycin, erythromycin, after front teeth, doxycycline (colors unerupted deciduous teeth). |
|
|
Pharengitis (children)
|
Epidemiology/Risks:
Sore throat with inflamed pharynx. Any age Want to r/o Group A strep Strep- School age child, some clustering in the fall/spring Does not ususlly follow URI Clinical: Who do we CX Fever of any degree, Pure or predominant complaint of sore throat (VERY PAINFUL) Maculopapular rash not around mouth. Absent congestion, everything else!!! Diagnostic: Only do throat cx if the clinical course is consistant. Antigen testing (if –'ve you still have to culture, if +'ve you have it) Causes/Tx Strep PCN 10 days Amoxicillin 10 days Time is b/c shorter course will not eradicate it, and many will have recurrence. Perhaps give script and have fam wait to fill until next day when cx comes back? |
|
|
AIDS associated PCP
|
@ risk with CD4 Below 200 cells/ml. The organism is a ubiquitous yeast.
Often subacute progressive symptoms including fever, tachypnea, and hypoxia. Laboratory data include elevated LDH (lactate dehydrogenase), increased A-a gradient (large difference between arterial and alveolar oxygenation) and decreased PaO2 CXR reveals batwing/butterfly infiltrate. Dx- Symptoms/Hx with definitiva answer via bronchoalveolar lavage and silver stain. Tx- TMP/Sulfa, Pentamadine, Atovaquone, or Clindamycin/Primaquine (or atovaquone) combo. Death of the organism casues a MAJOR immune response. If the A-a gradient is >35 or paO2<70 give the pt steroids...but ONLY if you are SURE it is PCP. Primary and secondary (post infection to prevent recurrance) prophylaxis is indicatd with CD4<200 using TMP/SMX. Once CD$>200, stop prophylaxis. |
|
|
AIDS Associated Cryptococcal Meningitis
|
@ risk with CD4 <100 cells/ml. Aquired by inhlaltion of the ubiquitous orgnaism. Causes fungemia and as it has a predilection for the CNS causes meningitis.
Symptoms: Fever, Headache, Meningismus (nuchal rigidity?), Vision/mental manifestations and rarely sking findings. Typical Crypto CSF includes Pleocytosis with predominatn lymphocytes, elevated protein, normal to slightly low glucose, elevated pressure...but may be normal. (Slightly high WBC/prot slightly low Glucose is classic crypto) Dx: Lumbar puncture with isolation. India ink is - 20% of the time, so use crypto antigen when possible. Tx: Amphoteracin B/Flucytosine combo. If ineffective, LP to releave CSF pressure. When symptoms improve change to fluconazole. Therapy should continue until CD4>100 for 3 months after the first 12 mos. Do not give primary prophylaxis, but 2ndary as above. |
|
|
AIDS associated Toxoplasmosis
|
The most common AIDS encephalitis. @ risk with CD4<100. 10-40% of the Us pop has it in latency, and up to 70% elsewhere, this is reactivation. Initial infection by the intracellular protozoan is via undercooked meat, inhalation of cat excrement, or transplacental. Reactivation is either in the Lung, Eye. or CNS.
Symptoms: H/A, confusion, fever, Focal neurological findings, Seizures. Generally onset is insidious and meningeal signs are rare. Dx: Usually presumptive based on radiography (multiple ring enhancing lesions in the brain on MRI...PET/SPECT with no incr uptake is consistant, with uptake --> lymphoma). Serology is also used, although IgG is sometimes - and not sufficient to pin the ilness on toxo. IgM is a sign of acute infection. Also remember that the immune system is waining. We may even see an entire hemisphere shift due to edema...bad. Tx: Pyrimethamine and sulfadazine with possible steroids for the edema. Continue therapy until CD4>200 for 3 months. If the pt does not respond in 2 weeks a brain biopsy is warranted. Prophylaxis with TMP/Sulfa is recommended with CD4<100 and + IgG. It can be stopped aboce 200 (decreases risk for PCP as well) |
|
|
AIDS associated CMV Retinitis
|
@ risk with CD4<50. Humn Herpes Virus 4/CMV Persists after primary infection and is shed intermittently. Over 75% of adults have it. Retinitis is bay far the most common (85%) manifestation, but GI/Pulm/Neuro are possible.
Symptoms: painless blurring/loss of central vision, floaters, or flashing lights. Retinal Infarct described as eggs and ketchup. Tx: Any pt with CD4<50 and symptms should see an opthalmologist, and should also be screened every 6 months. Gancyclovir (neutropenia) Gancyclovir Implant (Retinal detachment, infection) valgancyclovir (neutropenia) Foscarnet (Nephrotoxicity) Cidofovir (Nephrotoxicity) Tx should continue until CD4>100, Virus<50 and Opthomology exam demo no active disease. success rate is low. The Best TX is haart to improve the pt's immune system. Primary prophylaxis is not recommended. |
|
|
AIDS associated Mycobcterium Avium Complex (MAC)
|
@ risk with CD4<75. Ubiquitou organism in water and soil, it is often inspired or ingested and then spreads.
Symptoms: Fatigue, Weight loss, high fever, night sweat, watery/non-bloody diarrhea, but generally feel ok. Significant lymphadenopathy (abd/inguinal/axillary). Labs include decreases WBC (marrow infiltration). Dx: Growing the organisn in blood or marrow (not from sputum or feces as the bug is always there). We can Tx based on symptoms while we wait 2-4 weeks for the culture. Tx: Clarithromycin/ethambutol combo +/- rifabutin. Should continue until CD4>100 for 3 months after first 6-12. Primary prophylaxis is recommeded for CD4<50 |
|
|
AIDS Associated Malignancies
|
Lymphoma (esp in CNS) almost always associated Epstein Barr
Kaposi’s Sarcoma, HHV8 Cervical/Anal carcinoma, Human papilloma Hepatocellular carcinoma, Hepatitis B or C Multiple myeloma not due to a virus, Likely a result of effects on B cells… |
|
|
AIDS associated CNS Lymphoma
|
Essentially All Epstein Barr Related, usually in pt's wtih lower CD4 (avg 38) but possible @ any time. Manifestations are similar to toxoplasmosis/other focal lesions.
Dx- EBV PCR +, Negative Toxo titer, already on chronic TMP/SMX. SINGLE lesion, crosses the midline, Perifentricular location, SPECT/PET with increased uptake. Brain biopsy (if no change after 2 weeks of toxo tx). Tx: Antiretroviral therapy, high dose Dexamethasone and radiation. Survival is poor. |
|
|
AIDS associated Kaposi's Sarcoma
|
Due to infection with HHV8 (sexually transmitted affecting more HIV+ MSM than HIV+ IVDR). Leads to angiogenesis resulting in purple or red lesions most commonly affecting the skin (but can also affect Gi Mucosa of resp). Significant bleeding is possible.
Dx: Biopsy to rule out other lesions, often confused with bacillary antiomatosis (a lesion due to Bartonells Henslae (cat scratch fever). Tx: HAART, Local therapty (limited cutaneous disease), Radiation/Chemo for mucosal/disseminated KS. |
|
|
AIDS associated Progressive Multifocal Leukoencephalopathy
|
Caused by JC virus(polyoma virus) resulting in demyelination of nerve tissue.
Symptoms: Localized Neurologiacl findinge early on which may progress to more global symptoms. Lesions start as small foci that enlarge over time with minimal inflamation (no ring enhancement). Dx: Lesions appear white on T2 MRI but dark on T1. CSF is - but PCR for JC is +. Tx: HAART (but may not be effective) and antiviral therapy directe at JC including cytarabine or cidofovir. |
|
|
AIDS Dementia Complex
|
A syndrome of cognitive and motor dysfunction caused by HIV (itself).
Mild Form: Difficulty with concentration/Attn, Slowing motor activity wtih hyperactive reflexes and release reflexes (incl. snout response). CSF and MRI may be non-diagnostic. Severe ADC: Significant motor slowing with unsteady gait Patient’s alertness is preserved CSF is not diagnostic MRI shows atrophy and white matter changes Tx: Antiretroviral therapy with agents that penetrate the CNS. |
|
|
Slow Virus Disease
|
The phrase "slow virus" infections is a clinical concept and refers to infections of the central nervous system (CNS) with a long incubation period (months or years) followed by a prolonged clinical illness (many months). The infections though uncommon are usually fatal. Slow virus infections are on the rise due to increases in chronologically challenged, immunodeficiency, and "therapeutic misadventures."
Slow Virus Disease caused by conventional viruses: Progressive Multifocal leukoencephalopathy (PML) Subacute Sclerosin Panecephalitis (SSPE) Multiple Sclerosis (MS, Perhaps) Human Slow Viral Disease Caused by unconventional Virus: Creuzfeld-Jacob disease (CJD) Kuru Variant form of CJD (VCJD) |
|
|
Progressive Multifocal Leukoencephalopathy
|
Is caused by a small ubiquitous DNA virus JC (polyoma family, related to BK), the predisposing factor being immunosupression allowing the virus to replicate in the CNS. The incidence is greatest in older adults and HIV patients. The Virus has a bidirectional promoter, with the early genes coded in one direction, and the late in the other. Nearly 100% of people have it, and it spreads via urine and resp tract to close contacts. The infection is on the rise.
Pathogenesis: Upon infection JC immediately jumps to the blood and infects the kidney (hence shedding in he urine). The preceeding is often asymptomatic. Upon reactivation (Surgery, AIDS, age, cancer Tx, Integrins used to tx MS/IBS) it passes the BBB and causes PML, viral lysis/demyelination of the CNS as a result of the destruction of Oligodendrocytes. Dx- Generally made on autopsy, but at times in life. Signs/symptoms Internuclear inclusion bodies in biopsy material Histo stain with antibodies to polyoma. In situ hybridization with labeles/nucleic probes Tx: i. Antiviral therapy not very effective, we need to correct the immunosupression. HIV requires Haart therapy for this, Txplant we reduce antirejection drugs, etc… |
|
|
Subacute Sclerosing Panencephalitis
|
A CNS disease associated with measles virus reactivation in children. Has becomes less common with the introduction of an effective measles virus vaccine.
Pathology: Reactivation of a mutant measles virus. Hx of measles before 2 years, and then 6-15 years of asymptomology with latent virus (likely in the blood/lymphocytes, also possibly in CNS). Then we get immunosupression of some kind. It leads to reactivation and replication. It is most often seen in males and pregnant women. The virus then establishes an infection of the CNS and selects a mutant that is replication defective as a result of the interferon induced adenine deaminase (changes adenine to innosine, A to G). (in these pt’s you can’t replicate replication competent measles. Later immunity is restored and CTL’s kill infected neurons, so it is an immunological disease. The mutant measles makes LOTS of nuclear capsid. This leads to demyelination of the CNS leading to…. Symptoms- Begin with a gradual progressive phychoneurological degeneration, seizures, monoclonis jerks, ataxia, personality changes, occular abnormalities, spacticity, coma, death. Dx- The pathology is in the white matter of the stem and cortex, the inclusion bodies are visualized. The presence of antibodies to measles in CNS can be quite high. Replication competant virus is awfully hard to come by from these pt's. Tx- None. Ribavirin and Inf A are beind investigated experimentally. |
|
|
Prions
|
(pronounced Preeons)- Isoforms of the same endogenous protein one of which is pathonumonic. Prions made in the laboratory have been shown to be infectious in animals. Protein misfolding leads the formation of pathogenic proteins which are toxic to neuronal cells. Once formed the misfolded protein catalyzes its benign counterparts to follow suit, effective self replicating. These agents are notoriously hard to inactivate, even using UV, Heat, and Formalin. They cause Transmissible Spongiform Encephalopy (TSE) (like Scrapie, CJD, Kuru, and Mad Cow)
|
|
|
Kuru
|
The first human spongiform encephalopathy suspected of being infections. Originally seen in the Fore Tribe of New Guinea. Kuru examination, the incidence in 1960 was high and decreased in females and males.
Missionaries came in and convinced people that they should not eat the brains of their dead relatives (tribal custom) The females had more kuru in the first place as they were in the kitchen and thus were microinnoculated by bone fragments while preparing the meal, and everyone via consumption. It is transmissible spongiform encephalopathy. ESR like scrapie |
|
|
Creuzfeld-Jacob Disease
|
The prion is a ubiquitous protein (in every cell) which can misfold. This can be spontaneous, predisposed through genetics, or the prions can be introduced iatrogenically including corneal transplant, Dura Transplant, Growth Hormone etc, and less sterile instruments. Inoculation via consumption can also produce the disease, but more slowly (migratin time). The alteration is a change from Alpha helical to B-pleated sheets. When this happens you get prion corsslinking forming fibrils inthe brain which are toxic to neurons.
Symptoms- A. Ataxia, neurological signs, alterations in hearing, speech, vision leading to dementia. Same pathology as scrapie and kuru. Dx: E. Dx is via the presence of the protease resistant fibrils. When eaten it gets into the peyers patches, than in the PNS of the gut, then to the CNS, then the brain. The normal protein is expressed in all cells and so it spreads easily. No Tx? Mad COW (bovine Spongiform Encephalopathy (BSE)) is a variant of CJD and may be infections in humans. |
|
|
Heptatits A
|
AKA infectious hepatitis, Single Stranded + RNA non-enveloped enterovirus.
Transmission: Fecal-Oral route, contaminated food and water. Pathogenesis: The disease is worse in adolescents and older as the immune response becomes more competent. The incubation period is 15-20 days (short incubation hepatitis). Mortality <1% with carrier state uncommon. Symptoms: Acutely (A for Acute) Fever, Rash, jaundice, abdominal pain. They may go away in weeks. Dx: Antigens in the blood (serum agglutination?) Tx: Vaccine (very important), killed virus, generally with no complications. Attenuated live vaccines are still experimental. As a result of sanitation, many adults are HAV- (so outbreaks are possible and would be BAD), and need to be vaccinated before traveling to an area endemic for HAV. Note that there is an HAV/HBV combo now Immunoglobulin is highly effective prophylactically early in incubation s/p exposure. |
|
|
Hepatitis B
|
AKA Serum Hepatitis, the Dane particle; DS circular DNA enveloped (HbSag) virus with a special single stranded region. The inner nucleocapsid core is the HbCag (core antigen, including HbeAg). During replication, it integrates into a host chromosome is transcribed into HBV RNA, which is then reverse transcribed into DNA. This is unusual, and makes HBV susceptable to inhibitors of reverse transcriptase.
Transmission: Sex, Percutaneous, Blood. Asymptomatic carriers tend to spread infection (carrier rate is higher in poor/alcoholic/addict pop who often sell blood). Pathology- Long incubation heptatitis (48-180 days) with higher morbidity/mortality. 40% of thsoe infected (esp with young exposure) become chronic carriers. This has decreased wtih the vaccine. Symptoms- The most common outcome is asymptomatic infection. If symptomatic, Malaise and jaundice may take months to resolve. Chronic infection leads to hepatocellular carcinoma (due to replication moteif and regeneration) and cirrhosis/ascites (exacerbated by EtOH consumption). Dx- Presence of Surface or core antigens or antibodies in the blood. There is alot of incomplete assembly leading to high concentration of HbSag int he blood (Australian antigen). This is used to detect infection, carriers, and for vaccine. With similar symptoms, HAV and HBV are distinguished 1. Hx of transfusion within 48-180 days (B) 2. RIA 3. ELISA 4. Hemagglutination test Tx- Vaccine; Originally HbSag from blood (but transmitted HIV, clean versions are still around), so now we use Hep B antigen made in yeast (recombinant, used for high exposure risk like health care workers and institutionalized people). There is also an HAV/HBV combo now. It is not super antigenic requiring 3 injections. This is the first vaccine to prevent cancer in Humans. Regular gammaglobulin is ineffective, however Hyperimmune Globulin is protective for minimal exposures (no prot assoc with transfusion hepatitis). Infants of HBV+ moms are vaccinated within days of birth. Note that HBV vaccine/immunity is also protectie for Hep D. Interferon therapy may eliminate a carrier state (change to immune state). NRTI's are also used (RT step) |
|
|
Hepatitis C
|
AKA Non A-Non B, + RNA enveloped virus.
Transmission: Sex (less freq than B), blood, percutaneously, other undefined mechanisms. Due to the carrier state and testing for A and B, Hep C now acounts for 80-90% of all transfusion hepatitis. Pathology- One problem is the prevalence of the asymptomatic carrier state, so that the fist signs may be cirrhosis, hepatocellular carcinoma, or B-cell lymphoma decades after exposure. Nte that the mechanism of oncogenesis involved inflamation and free radicals with liver regeneration, so it differs from HBV. Symptoms- 70% are asymptomatic. The clinical picture and epidemiology are as Hep B. Dx- Via antigens and Antibodies in the blood. There may be many strains as the first tests only ID about 60% of Non A-Non B hepatitis, the other 40% are labeled as Hepatitis E. Tx- vaccine is still in the experimental stages. Interferon Therapy with Ribavirin can be effective. |
|
|
Hepatitis D
|
AKA Delta Agent, A circular - RNA enveloped virus encoding one open reading frame generating 2 proteins (including its capsid, the delta antigen...has a resemblance to plant viroids). It requires HbSAg as its envelope, so that only those with HbSAg in their blood are susceptable.
Transmission- Percutaneous, sex, blood, person-person contact. Pathology- Fulminating, and life threatening. It is cytolytic to hepatocytes and replicates like crazy. It can also persist in the chronic form. Symptoms- Fulminating (BAD fast) in duel B/D infections or with D infection in chronic B carriers. Severe and rapidly progressive iver disease. Dx- Hep D surface antigen Tx- The hep B vaccine is protective. |
|
|
Hepatitis E/G
|
HEV
Single stranded + RNA non enveloped virus. Hepatitis E, which is distinct from HAV, is transmitted via the fecal oral route and is related to other enteric viruses. Pathogenesis- HEV causes significant morbidity and mortality in pregnant women (including fetal demise) in India and parts of Asia. HEV is rarely encountered in the U.S., however travelers to HCV endemic areas may bring it back (don't drink the water). symptoms- Similar to Hep A Dx- HeSAg Tx- vaccine in the experimental stages. HGV: Recently another hepatitis virus was isolated which is related to HCV which is also transmitted percutaneously. This virus HGV appears to be only occasionally associated with human disease. |
|
|
Primary Peritonitis
|
AKA spontaneous Bacterial peritonitis (SBP)- Infection not related to abnormalities of the abdomen. Infection of fluid that is present (ascites) most commonly a result of cirrhosis, but also malignant disease, CHF, or Lymphedema.
Bacteriology: Enterobacterecia spp. (69%) Escherichia coli Klebsiella pneumoniae Enterobacter Streptococcus pneumoniae (5-15%- likely the pt has bacteremia due to upper resp inf) Staphylococcus aureus (2-4%-comes in from the skin) Anaerobes- rarely, and if present, usually with another organism. Cause- Migration through Gi tract into ascites (party time), but also perhaps Hematogenous or Lymphogenic spread of bacteria. Symptoms: fever Abd pain (diffuse) Abdominal Tenderness (pain on palpation) Diarrhea Hypo-active bowel sounds present. Dx: Ascites is present, paracentesis should be done. Look at cell count (white cells) albumin/protine, Culture/stain. Leukocyte counts greater than 300 WBC with Polymorphonuclear (PMN) cell count greater than 250 cells. If the Id of cells is different, the infection may not be bacterial. An albumin gradient >/= 1.1 (serum - ascites) leads to a dx of portal hypertension, if <1.1 it is consistent with SBP but not diagnostic. Fluid CX and Gram Stain- Cultures are + 80% of the time. If the fluid has >250PMN's and - CX, we should still tx. TX-Start with a 3rd gen cephalosporin (ceftriaxone) for 10-14 days. If Cx is + antibiotis can be changed for the specific organism. Additinally the paracentesis is doen therapeudically to ease breathing as the ascites will not generally self resolve (but beware of hypovolemia) Proplhylactic tx- A pt with ascites is less likely to be infected if antibiotics are given, but the bugs that do get though are worse/resistant, and there is no effect on survival. |
|
|
Secondary Peritonitis
|
caused by a spillage of GI/GU organisms into the peritoneal cavity. Causes of spillage are many/varied including perforation, ischemia, organ rupture etc.
Bacteriology: Multiple normal flora including GI- E. coli Enterococci Anaerobes such as Bacteroides fragilis GU- E. coli Anaerobes including B. fragilis and gram positive anaerobes Streptococci Pathology: Significant local inflammatory response- outpouring of fluid, high protein, large # of white cells, fibrin. The result may be containment and resolution, walling off creating an abscess, or a systemic response and spread/sepsis. Systemic resposne includes release of TNF-A, IL1, IL6, INF-G resulting in paralysis of the gut, low renal perfusion, Increased CO, venous dialation and hypoxemia leading to shock and death. This is one of the most common causes of sepsis for inpatients(AKA abdominal catastrophies) Symptoms: SEVERE abdominal pain N/V Fever tachycardia Hypotension Abdominal rigidity (AKA acute abdomen) Dx- Supine, and Lat decub radiographs to look for free air or dialated loopes of bowel CT to check for the location of the fluid collecion/locate the lesion. Tx- SURGERY to correct the initiating problem, drain any abscess broad range polymicrobial therapy (Piperacillin/Tazobactam, Ampicillin/sulbactam) |
|
|
Peritonitis from peritoneal dialysis
|
There is a catheter that is placed in the abdomen for daily dialysis, may lead to colonization and therefore infection.
Bacteriology: The organisms causing this infection are usually skin colonizing organisms such as Staphylococcus epidermidis, Staphylococcus aureus, and Streptococcus spp. Other less commonly isolated organisms are the gram negative enterics and yeast symptoms: Abdominal Pain N/V Fever Diarrhea Dx: Dialysate has increased number of leukocytes and is cloudy. Cultures are positive about 90% of the time. Tx: Empiric antibiotics usually include vancomycin to cover the gram positive organisms. The catheter should be removed, but not necessarilly in all infections. |
|
|
Intraperitoneal Abscess
|
Can be 2ndary to secondary peritonitis resulting from Appendicitis
Diverticulitis Pancreatitis Perforated ulcers Trauma Previous abdominal surgery (most common) Bacteriolgy: Typically polymicrobial: Gram negative enterics Anaerobes Enterococci Symptoms: High intermittent fevers Shaking chills Abdominal pain Tenderness over the area of the abscess Dx: Ct will reveal abscess Tx: The main therapy is drainage. Drainage can be accomplished by radiology or by surgery (the former is better). Antibiotic therapy is similar to that mentioned for secondary peritonitis. |
|
|
Acute Appendicitis
|
Inflamation of the Appendix.
Symptoms: right lower quadrant pain anorexia nausea and vomiting low grade fever rebound tenderness guarding Clinical findings are non specific and may be misdiagnosed. Dx: radiology is not specific/sensitive. Perforation will remove pain but leads to abscess, Tx surgery Antibiotic therapy similar to peritonitis |
|
|
Diverticulitis
|
Diverticula are herniations of the mucosa and submucosa of the colon. They are usually located in the sigmoid and descending colon and are more common in older people. Inflammation occurs in about 20% of diverticula.
Perforation of the inflamed diverticula occurs rarely. Symptoms Identical to appendicits but inthe lower left quadrant. Dx- CT, Barium enema, Colonoscopy Tx- Antibiotics similar to Secondary peritonitis for abou 14 days. Surgical drainage only in the case of a very large abscess (>5cm) |
|
|
Visceral Abscesses
|
can be Pancreatic, Hepatic, or Splenic etc. They can be bad, and are seen via CT scan.
Again Tx may include drainage, repair, and possible antimicrobial therapy. |
|
|
Cholecystitis
|
Inflamed gall bladder disease (many are not inflamed/asymptomatic). Gallstones in the cystic duct leading to obstruction (may be partial) and an increase in the intra-ductal pressure cause distention of the gallbladder.
Tissue necrosis and proliferation of bacteria leads to cholecystitis in about half of the patients, perforation is rare. Bacteriology: The most common bacteria are E. coli, Klebsiella spp., Enterobacter spp., and Proteus spp. Occasionally other bacteria may be present Symptoms: Fever Nausea and vomiting Right upper quadrant pain to palpation (Murphy's sign [press on RUQ, ask to inhale, pt stops b/c of pain]) Icterus/jaundice is not very common Normal liver function tests may be present, or slightly elevated. Dx: Ultrasound will see the stones as well as the thickening of the wall and the fluid leakage (pericholycystatic fluid. Also hepatobilliary Iminodiacetic acid scan (HIDA scan) discerns if the gall bladder fills with the radio-dye (if not --> obsctuction). Tx: Antibiotics in the case of perforation. If acute perforation occurs an invasive laporotomy will be indicated. If perforation is absent (uncomplicated acture cholesystitis) the pt needs to stop eating fatty foods to let it rest). Then when inflamation is gone we can do the elective cholecystecomy laproscopically. |
|
|
Cholangitis
|
Gall Stones in the common bile duct/billiarty tree cause backup and infection in the billiary tree (not galbladder iteself).
Bacteriology: gram negative enterics Symptpms: fever right upper quadrant pain prominent jaundice possibly shock, may appear septic Charcot’s triad - fever, chills and jaundice Tx: Immediate antibiotics are necessary Decompression of the common bile duct is necessary by either surgery or by esophago-retrograde-cholangio-pancreatography (ERCP- you fetch the stone or place an opening stent). |
|
|
Enteric Viral Disease
|
Enteric Disease is a common cause of death in children under 5 throughout the world. Of the 10.5 million under 5 death, diarrheal disease falls 3rd behind Perinatal problems and ALRI. Other major players include malaria, measles, congenital defects, HIV, and Pertussis, although some of these are changing with vaccine availability.
Enteroviruses (picorna virus group, diarrheal disease) Poliovirus types 1,2 and 3 Coxsackieviruses (Group A) Types 1-23 Coxsackieviruses (Group B) Types 1-6 Echoviruses Types 1-31 Enterovirus Types 68-72 Rotaviruses (riovirus group, Diarrheal disease) Norwalk Virus (Caliciviruses)and Norwalk-like Agents (Astroviruses) |
|
|
Enterovirus (Picorna Virus) general Info:
|
Single Stranded + RNA non-enveloped viruses resistant to inactivation by detergents, room temp, and acid pH (sensible with GI invasion). They produce world wide epidemics. Polio and coxackie B are the major players.
Transmission: Fecal-oral route. Pathogenesis- Multiply in the GI tract. Occuring predominantly in kids, subclinical infection is more common than those recognized. Incubation period of 2-10 days with sommunicability 2-3 days PRECEEDING symptomology, and continuing for days/weeks after resolution. Day 1- Primary replication occurs in epithelial cells and lymphoid tissues of the throat (tonsils) and intestine (Peyer’s patches). Day 2: Virus spreads to regional lymph nodes Day 3: Viremia carries virus to sites of secondary multiplication, first symptoms Day 3-7: Second viremia occurs leading to infection of target organs, additional symptoms. If the virus does not get to the target organs you prevent the disease, so we want to hit the bug in the blood by raising antibodies, likely by vaccine. Symptoms: vary wiht age of the pt. |
|
|
Polio
|
Franklin Roosevelt had it and drove the creation of the march of dimes and NFIP. (national foundation for infantile paralysis). Salk developed the inactivated virus, Sabin the attenuated oral. Next for elimination by WHO, targeting India, pakistan, nigeria, bangladesh (america, europe and western-pacific regions are all polio free).
Pathology: In young children 6 months - 1.5 years old (as with poor sanitation in the early 1900's) symptomolgy is relatively benign as their nerves do not yet have viral receptors. This conferred immunity. As sanitation improved older kids got it (often from swimming pools) leading to sudden fever and paralysis. 3 kinds of clinical manifestations: Abortive Poliomyelitis: (most common form) subclinical/mild illness which includes fever, malaise, nausea, headache, sore throat etc. Mistaken for a cold. Nonparalytic Poliomyelitis (Aseptic Meningitis)- additional symptoms include stiffness and pain in the back and neck Paralytic Poliomyelitis (5% of cases)- flaccid paralysis which may involve all 4 limbs or brainstem, paralysis of cranial nerves and muscles of respiration. Liklyhood is increased by age, gregnancy, tonsillectomy, trauma, fatigue. Dx- Isolation of virus from throat swabs early after onset (resuls in 4-8 days). PCR for CSF (results in 1-2 days) as well as general testing ( Incr WBC (Neutrophils --> Mononuclear lymphocytes), Incr protein, Normal sugar, . Serology for antibodies to determine acute vs. convalescent levels. Tx- Back in the day, The Iron Lung was used to help breathing. Today we vaccinate with live-attenuated tirvalent or Killed virus to block the viremia steps. (oral no longer recommended as it caused 6-8 cases/year). The oral vaccine does give life long protection, and is shed in feces, spreading immunity to close contacts. Enteric Precautions including thorough handwashing. |
|
|
Coxsackievirus
|
Large subgroup of Enteroviruses divided into two subgroups (A [1-23] and B [1-6]) based on pathogenic potential in mice
Transmission: Fecal-Oral route Pathogenesis: Incubation Period: 2-9 days Virus found in blood and throat early in disease, and in the feces 5-6 weeks after appearance of symptoms Symptoms: Coxsackie B viruses are the most common cause of viral heart disease Herpangina (painful mouth infection) Aseptic Meningitis Hand, Foot and Mouth Disease Myocardiopathy Acute Hemorrhagic Conjunctivitis |
|
|
Echoviruses
|
Enteric Cytopathogenic Human Orphan (ECHO) virus. There are over 30 serotypes, some of which infect the human alimentary tract.
Pathogenesis is similar to other enteroviruses. The virus is recovered from the throat and feces in most infection and the CSF in cases of aseptix meningitis. It should be considered for outbreaks of aseptic meningitis or febrile illness with rash, both in the summer; or outbreaks of diarrheal disease in young infants not infected with enterobacteria. Symptoms: No symptoms for many infections Aseptic meningitis Maculopapular and vesicular rashes Conjunctivitis Diarrhea |
|
|
Enteroviruses 68-72
|
Enteroviruses that are not polio/coxsackie/echo are numbered.
Enterovirus type 68- isolated from the respiratory tract of kids with bronchiolitis or pneumonia Enterovirus 70- chief cause of hemorrhagic conjunctivitis; highly communicable Enterovirus 71- major cause of central nervous system disease; also causes hand, foot and mouth disease Enterovirus 72- same as Hepatitis A Don't worry about specific symptoms, but knwo general information about the group. |
|
|
Rotavirus
|
Double stranded DNA Non-enveloped virus with a wheel-like appearance(hence the name) and complex architecture. # serogroups and 4 serotypes Id'd. Animals and Humans are both infected, however they do not cross from one to another.
Transmission: fecal-Oral. It is environmentally stable and resistant to handwashing agents. Susceptable to 95% ethanol, Lysol, and formalin. Pathology: the SINGLE most important world wide cause of childhood diarrhea (similar %age of Gastroenteritis/infantile diarrhea in developed/developing countries!!). Affects most children <4 y/o, predominating in the winter (nov-apr in US, earlier/longer/attenuated severity in west), with outbreaks in daycare centers, hospitals and camps. Incubaiton in 1-3 days, shed for 2-12 days after symptoms. In kids uner 6 months and adults, it is asymptomatic. Symptoms: Abrupt onset of vomiting, diarrhea, low-grade fever Cough and runny nose in 50% of young children Dehydration and acidosis in severe, life threatening cases (requires hospitalization) Dx- Viral isolation is not practical as it does not grow in culture. Detected in feces by EM/Elisa IgG is produced and persists for years, but serology is not useful in disease management. Tx: Live trivalent vaccine (rotashield) was immediately removed from the market due to intussusception. A new vaccine is now out called RotaTeq. |
|
|
Norwalk Virus (Caliciviruses) and Norwalk-like Agents (Astrovirus)
|
Norwalk infections (epidemic non-bacterial gastroenteritis including diarrhea) are seen later in life (50% of adults over 50 have antibodies). There are at least 3 serotypes. Astrovirus was detected by EM in '75 described as "norwalk-like" virus but is immunologically distinct. There are 8 human serotypes.
Pathogenesis- Incubation perios is 10-51 hours (CHARACTERISTIC, possibly after consuming infected food). Virus is detected in stool at rapid onset of symptoms. Clinical course lasts 24-48 hours and rarely requires hospitalization. Frequently it affects passengers on cruise ships, and can be in buffet's (salads, sandwiches, produce/fruit, meat, fish, baked goods, oysters, greenonions, shellfish). Symptoms: Diarrhea Nausea Vomiting Low-grade fever Abdominal cramps Headache Malaise Dx- There are many methods of detection, but only cell culture isolation and antibody assay test infectivity. PCR and Cell culture are the most sensitive, but are of little use on a cruise ship. Tx: Hand washing/sanitation/disinfectant stations, safe drinking water and food. |
|
|
Ex Vivo Gene Transfer
|
Indirect method of gene transfer:
transfer of genetic material to cells located outside the host (remove cells, intro in the lab, culture the cells and be sure that the gene took, reintroduce them to the host. Good for hematopeotic issues) |
|
|
In Vivo Gene Transfer
|
Direct method of Gene Transfer:
transfer of genetic material to cells located within the host (infect the pt with the virus) |
|
|
Clinical Applications for Gene Therapy
|
1. Single‑gene inherited disorders:
Simple diseases with a clear target whose alteration corrects the pathology. Includes Sickle Cell, Hemophilia, Inherited Immune Deficiency (deaminase deficiency), Cystic Fibrosis, Hypercholesterolemia, LDL defects. 2. More common multifactorial disorders: Several genes are involved in these diseases like coronary heart disease and diabetes. The approach needs to be assessed, how many genes do we send in, can a cascade effect be used? 3. Cancer (currently most clinical trials are based here): A multistage process driven by either somatic mutations (inherited or novel) followed by clonal selection or germline mutation of tumor supressor/DNA repair genes. Less complex than multifactorial disease as we know the process and how to alter it. 4. Infectious diseases: Many chronic ilnesses are potential targets including herpes and hepatitis, but HIV has gotten more attn. 2 main approaches- Post exposure vaccination to boost host immunity Pre-exposure expresson of genes rendering host cell immune including antagonists for viral proteases, viral receptor antagonists etc...as well as boosting host immunity wity cytokines etc. |
|
|
Principals/Technical problems with Viral Gene Therapy
|
Vectors are usually disabled/altered viruses or plasmids with a lipid coating (less immunogenic). Common vectors include retroviruses, adenovirus, Adeno-associated virus, Herpes simplex virus, and vaccinia (pox).
1. Gene Identification and Cloning (can we fit it in the virus) 2. Identification and access to the Target Cell (easy in diabetes, tougher in more complex diseases where the target may be less well known or harder to access) 3. Efficiency of Gene Transfer (how much virus to use, can we alter it?) 4. Duration of Expression 5. Repeat Dosing (can be a problem if we have developed an immune response to the earlier innoculation) 6. Safety 7. Clinical Endpoints (what is a cure?) 8. Disease Pathophysiology |
|
|
Rhabdovirus (Rabies)
|
Bullit shaped - RNA enveloped virus with a Glycoprotein G on itse surface. That "G" protein recognized ACh receptors allowing for the ready infection of the CNS (pathogenicity). The - RNA is translated into varying length +RNA and full length RNA to be copied as well forming a complex structure called the Negri Body (pink with a blue center), Diagnostic for rabies.
Epidemiology: Carried most often by Carnivorous wild animals (especially skunks, raccoons, foxes, coyotes, and bobcats) and bats. Locally in the US Dogs and Cats are far less likely to have it due to immunization practices, as are other rodents and lagomorphs (rabbits etc). Pathology: The virus infectes broken skin or mucus membranes via innoculation (animal saliva, airbourne, note human saliva is not infectious). It infects the epithelial cells for a month to a year (the eclipse period) after which it jumpts to unmyelinated motor or sensory nerve ends. The former produces tingling/parasthesis, and the latter a loss of motor control. If we prophylax and kill it before it jumps we are ok, after it jumps the game is over. In the CNS it does not kill, but usurps machinery resulting in loss of fxn. Specifically it likes the transcriptosomes of the limbic system, cerebellu, and hippocampus, ocupying the ACh receptors and altering transcriptional patterns.. Outward migration from the CNS leads to the involvement of other organs. This may take months and be the first sign of rabies (hence organ donors may not know they are +). Dx: Histo staining for Negri body is the gold standard. Also fluorescent antibodies and PCR (the most sensitive but gives false +'ves). It can confirm rabies of unusual presentation. 50% of people bittn develop rabies, so there may be some effective immunity, we are not sure. |
|
|
Rabies (Symptoms, Tx)
|
Symptoms:
Raging rabies (furious) is easier to diagnose than paralytic (dumb) and other presentations are even more difficult based on signs and symptoms. Furious (encephalyitic) rabies: Parasthesia/itching at the site of infection Encephalitic phase including fever, terror, excitement, irritability, and aggressive behaior Raging phase- brain stem destruction, hydrophobia, foaming at the mouth, death (one way progression from bad to worse with the pt remaining aware until near the end) Paralytic (dumb) rabies: Paralysis (Hx of exposure being the real clue). can be mistaken for Guillan-bare Note that duel infection of both sensory and motor nerved may lead to extreme furious forms. Tx Options: Killed vaccines require 7-10 days to develop immunity and last for up to a year, called the Human Diploid Cell Vaccine (HDCV). immune globulins give immediate passive immunity with a half life of about 21 days. Rabies Immune Globulin Human (RIG) is IgG concentrated from plasma of hyperimmunized donors. Anti rabies serum, Equine (ARS) is refined concentrated serum from hyperimmunized horses. Considerations for Tx: 1. species of animal- carniverous wild animals and bats are the most likely infected. 2. Circumstance of exposure- unprovoked attacks are more suspect (than if you are trying to pet or feed the animal etc). 3. Type of exposure Bite- penetration fo the skin by teeth (all should be throughly washed and duel treated if indicated) Non-Bite Scratches, abrasions, open wounds, mucus membranes contaminated with saliva. Indications: For Dogs and cats: If healthy and observable for 10 days, observe and wait for Tx. If rabid or suspected, Tx If unknown/escaped check wtih pub health. For Wild Skunk bat, coyote, raccoon, bobcat, or other carnivore: Regard as rabid and tx unless proven negative by lab tests. For livestock. rodents and lagomorphs: Consider individually and consult public health. Tx is RARE in these cases. Pre Exposure Prophylaxis Mostly for high risk groups to provide protection for those with inapparent exposure, to protect those for whom tx might be delayed, and for those with documented exposure to attenuate the still necessary post-exposure prophylaxis. Post Exposure Prophylaxis Aggressive antivirals and lower body temp. Wash infection site with soap and water. Vaccinate according to schedule Concurrent Human Rabies Immune Globulin Tx may increase effectiveness of vaccine (1/2 in infection site, 1/2 in nearest lg muscle). If the person has been vaccinated, do not use RIG, give 2 doses of HDCV. If the person was vaccinated but the full regimen was not followed or if their immune status is not known, start with the full RIG + 5 HDCV doses, however if a pre tx appropriate titer is evaluated you may stop after 2. Booster Doses of Vaccine- For those at risk for exposure, titers should be checked every 6 months and booster given when indicated. If exposure is still possible but less likely, a longer time between tests may be permissable. |
|
|
Arboviruses
|
They cause severl hundred cases of encephalitis in the US/Year. High morbidity/Mortality. Inexperienced lab techs should not attempt to isolate teh virus as lab infections are fairly common.
They are single stranded linear + RNA viruses. Epidemiology- Characteristically they have a natural host, and travel to new natural hosts, or other species (like Us) via insectes. Generally they only cause disease during those times of the year when the vectors and people are in close contact, during the late summer/early fall. This may be monitored using sentinel animals. People are infected in 3 circumstances- 1. a result of travel to areas infested with vectors 2. amplification of vectors or natural host population (Seasonal for instance) 3. Environmental change causing migration (seasonal, climate change, pollution etc). Human - Human infection is rare as the viremia is low. Symptoms - See individual viruses. Dx- Often PCR is used. Comparison of acute phase and convalescent sera for antibody titers is critical The travel history of the pt is often significant. Tx Depending on the state of sentinal animals, mosquito abatement programs may be used. Local Arboviruses (in order of frequency) West Nile California (La Crosse) St. Louis Western equine Eastern equine (Most potent morbidity/mortality, controlled via mosquito abatement) Colorado tick fever |
|
|
Arenaviruses
|
AKA rodetn borne Hemorrhagic fever, Hemorrhagic fever virus (HFV). Characteristically can infect rodents (vector) and are transmitted to people via feces/urine aerosolized during cleanup. Also has an unknown natural host, perhaps monkey. Often with arboviruses b/c of extreme pathogenicity and that groups working on one are crazy enough to work on the other.
Transmission: via rodent excrement as above Pt blood, body fluids. Symptoms- abrupt onset with severe frontal and temporal headaches high fever generalized pains in the back. Diarrhea macular papular rash severe bleeding from nose, gums and genital tract gut and lung congestive with blood. Generalized hemorrhagic syndrome Tx: Ribavirin, Fluids, Hyper-Immune globulin Examples of HVA: Hanta virus Filoviruses (named because of their long filamentous appearance when viewed by electron microscopy) - rodent-borne HFV in travelers Marburg Ebola - brief but catastrophic self-limiting outbreaks in humans and great apes in equatorial regions of Africa. |
|
|
West Nile Virus
|
Arbovirus
a. Can range from an unapparent infection to a viral encephalitis characterized by profound muscle weakenss progressing to coma and death via multiorgan failture. Signs include: a. Fever b. Chills c. Vomiting d. Headache e. Profound Muscle weakness (can be Dx helpful) f. Respiratory failure/paralysis in severe Dx- Based on signs/symptoms and serology (antibodies) 3. generally you need an underlying medical impairment to cause severe disease, like age, diabetes, etc…Transplant pt’s are immunologically suppressed etc. It generally likes to infect crows. Natural hosts either adapt or die, and those that adapt are not too infectious. Human occurs when we get in the way of the normal Bird - Vector - bird cycle. |
|
|
California Encephalitis
|
Arbovirus
a. 2nd most common arboviral disease b. Found in young children c. Reservior is small rodents and rabbits d. Vector is woodland mosquito, so playing kids are exposed e. Develops mild illness H/A, vomiting, focal neurological signs. Morbidity/Mortality is low. |
|
|
St. Louis Encephalitis
|
Arbovirus
Signs: fever abnormalities in coordination photophobia encephalitis coma High morbidity/mortality. Dx is via serology (antibodies) |
|
|
Western Equine
|
Arbovirus
Signs: fever abnormalities in coordination photophobia encephalitis coma High morbidity/mortality. Dx is via serology (antibodies |
|
|
Eastern Equine
|
Among the most potent arboviruses
Signs: fever abnormalities in coordination photophobia encephalitis coma High morbidity/mortality. Dx is via serology (antibodies |
|
|
Colorado Tick Fever
|
Arbovirus
Colorado tick fever is Comparatively Mild It is the only one transmitted by ticks in North America. Presents with Fever Rash etc... |
|
|
Dengue Fever (breakbone fever)
|
Arbovirus
The most common arboviral infection in the world, the name means evil spirit in suahili. Environmental change may contribute to increases in incidence and wider mosquito vector distribution, and epidemiology has stood inteh way of vaccine development. Epidemiology: The only arbovirus with a human reservior (mosquitos are still the vector). There are 4 serotypes. If an individual contracts multiple serotypes at once you get Dengue hemorrhgic fever as well as dengue shock syndrome. Infection with the first serotype resolves and leaves antibodies (or Mom gives antibodies. When the 2nd serotype arrives, the antibodes cannot kill, but can opsonize the virus. Any cells with Fc receptors then take the virus up and it replicates like crazy. The preexisting antibodies also activate complement leading to cytokine strom, DIC, and all the bad stuff. Symptoms (systemic fever): Headache Stiffness Malaise Sudden onset of retroorbital pain EXTREME pain in the muscles and joints of the extremeties (This is the hallmark) Tx- No vaccine |
|
|
Yellow Fever
|
Still epidemic despite a vaccine. The vaccine is live attenuated virus so it does not travel well enough (yet has saved millions of lives, more than any other vaccine and RARELY cause complications).
Type I- In the Jungle you have Jungle yellow fever. More prevalent in SA, Carribbean, Africa. It spreads from Monkeys to humans. Type II- Is urban yellow fever which spreads from Humans to mosquitos, and back to humans. Both kill liver cells and cause jaundice, hence "Yellow" fever. |
|
|
Hanta Virus (Sin Nombre Virus)
|
Hanta virus causes an acute adult respiratory distress syndrome as well as hemorrhagic fever with renal syndrome. If not diagnosed early a high fatality rate results. The virus is present in wild mice and spreads to humans via inhalation of urine or feces.
Symptoms: It is often misdiagnosed... It begins with a prodromal syndrome, 3-4 days with general abrupt onset fever, malaise, myalgia. By 14-17 days post exposure, anorexia, nausea, vomiting…A pt may seek medical care at this piont but is often sent home after palliative therapy. By now we need to know, if we fail at this point it is quickly followed by the third step including cardiopulmonary involvement, GI manifestations like bleeding, low platelts, abnormal blood pic. 30-40% die within 24-48 hours of readmission. Hanta is big in some parts of the US. Mice are the host. Ex…someone has a cabin in the poconos, and the white footed mouse gets in and makes feces trails, so as we sweep the cabin out we aerosolize the dung in dust and inhale it. |
|
|
lymphocytic choromeningitis virus LCMV
|
Arenavirus- Rodent-borne viral disease. Causes Lymphocytis Choromeningitis, a mild afebrile diseas in children (they crawl on the floor and inhale mouse excrement...it can be severe on occasion). It may also be transmitted via organ transplant causing:
Fever Viral Syndrome Donor Organ dysfunction Multi Organ Failure Death. Reduction of immunosupressive therapy may be helpful |
|
|
Lassa, Marburg, Ebola
|
They are all in africa, and can Spread from Humans to Humans. They are deadly, so patients are kept in isolation. You breathe in excretions from the rodents. You hemorrhage to death. Kills endothelial cells, you bleed from every orophace.
Lassa fever is also seen in travelers returning from endemic areas. Marburg is endemic in central africa in villages, travels, and lab workers making vaccines from infected monkey cells. Ebola causes catastrophic infetin in humans, often transmitted to healthcare providers and a potential biological weapon. Self limiting as they kill so fast and completely that they kill off their hosts and then are gone. |
|
|
Filoviruses
|
Arena Viruses
Deadly HFVs with high mortality in central Africa. Infects great apes and humans from unknown animal reservoirs Includes Lassa, Marburg, and Ebola |
|
|
What do you need to know to develop a successful viral vaccine?
|
1. Major site of viral infection
Via mucosal sites of resp and GI tract Via mucosal surfaces followed by systemis spread via blood and/or neurons. Infection via needles or insect bites followed by spread. 2. Viral antigen(s) that elicit neutralizing antibody 3. Cell surface antigen(s) that elicit neutralizing antibody 4. The site of replication of the virus |
|
|
Problems in vaccine development
|
1) Different types of virus may cause similar diseases--e.g. common cold
2) Antigenic drift and shift 3) Large animal reservoirs. 4) Integration of viral DNA. 5) Transmission from cell to cell via syncytia. 6) Recombination and mutation of the virulent strain or of the vaccine virus. |
|
|
Live Attenuated Viruses
|
Advantages of live, attenuated vaccines
1)Activates all phases of immune system. Can get humoral IgG and local Ig 2)Raises immune response to all protective antigens. Inactivation, such as by formaldehyde in the case of the Salk vaccine, may alter antigenicity 3)More durable immunity; more cross-reactive 4)Low cost 5)Quick immunity in majority of vaccines 6)In case of polio and adenovirus vaccines, easy administration 7)Easy transport in field 8)Can lead to elimination of wild type virus from the community Disadvantages of live, attenuated vaccines 1) Mutation; reversion to virulence (this is a major disadvantage) 2) Spread to contacts of vaccinee who have not consented to be vaccinated (could also be an advantage in communities where vaccination is not 100%) 3) Spread vaccine not standardized--may be mutated 4) Poor "take" in tropic 5) Problem in immunodeficiency disease. |
|
|
Killed Inactivated Viruses
|
Advantages of killed, inactivated vaccines
1)Gives sufficient humoral immunity if boosters given 2)No mutation or reversion (a big advantage) 3) Can be used with immuno-deficient patients 4) Sometimes better in tropics Disadvantages of killed, inactivated vaccines 1) Many vaccinees do not raise immunity 2)Boosters needed 3) No local immunity (important) 4) Higher cost 5) Shortage of monkeys (polio) 6) Failure in inactivation and immunization with virulent virus. |
|
|
DNA Vaccines
|
1) Plasmids are easily manufactured in large amounts
2) DNA is very stable 3) DNA resists temperature extremes so storage and transport are straight forward 4) DNA sequence can be changed easily in the laboratory. This means that can respond to changes in the infectious agent 5) By using the plasmid in the vaccinee to code for antigen synthesis, the antigenic protein(s) that are produced are processed (post-translationally modified) in the same way as the proteins of the virus against which protection is to be produced. This makes a far better antigen than, for example, using a recombinant plasmid to produce an antigen in yeast (e.g. the HBV vaccine), purifying that protein and using it as an immunogen. 6) Mixtures of plasmids could be used that encode many protein fragments from a virus/viruses so that a broad spectrum vaccine could be produced 7) The plasmid does not replicate and encodes only the proteins of interest 8) There is no protein component and so there will be no immune response against the vector itself 9) Because of the way the antigen is presented, there is a CTL response that may be directed against any antigen in the pathogen. A CTL response also offers protection against diseases cause by certain obligate intracellular pathogens (e.g. Mycobacterium tuberculosis) All of the above means that DNA vaccines are cheap and therefore likely to be developed against pathogens of lesser economic importance (at least to drug companies) Possible Problems with DNA Vaccines 1) Potential integration of plasmid into host genome leading to insertional mutagenesis 2) Induction of autoimmune responses (e.g. pathogenic anti-DNA antibodies) 3) Induction of immunologic tolerance (e.g. where the expression of the antigen in the host may lead to specific non-responsiveness to that antigen) |
|
|
What are the 3 kinds of cell lines used in the Shell test
|
a) primary cells, which are directly explanted from animal tissue and have a short cell life,
b) diploid cells which have a normal diploid karyotype and can last from 5–50 cell passages, and c) heteroploid cells which are cancer cells which can last in vitro indefinitely. |
|
|
5 Major stages of Rashes
|
Macular- Red rash
Papular - Red raised, with clear fluid vesicular- Produces vesicels of fluid (more of a lesion, more fluid producing material). Pustular- Purulent vesicles Crusting- |
|
|
CMV Tests
|
Isolation from Urine- Only useful if the specimen if obatained from a newnate during the first several days of life indicating congenital infetion.
Use of Bronchoalvrolar lavage, and peripheral white cells is common. Shell tests: Centrufuge specimen onto a cellmonolayer on a coverslip in the bottom of a shell vial. Stain for viral antigen with monoclonal antiboyd after 1-2 days of incubation. PCR is also coming into favor. |
|
|
2 stages of Diagnosis
|
1. isolation/detection/Identification
2. Once a presumptive identification is made, then a confirmatory diagnosis can be made usually requiring the use of a specific immunologic method using antibodies of known specificity |
|
|
Syphilis (General, Dx, Tx)
|
A Chronic systemic disease caused by treponema Pallidum (the only of the 4 tremonemae sexually transmitted.. Humans are the only natrual host. We can develop cross reating non-specific nontreponemal antibodies, likely a result of the plasma membrane integral proteins.
Epidemiology: It has been on the decline in general but there has been an upturn int he last few years, with 2-3 times as many cases possibly going unreported. The male to female ratio is 2:1, and most often infection occurs in 20-14 year olds in urban settings. The most important source of early syphilis now is homosexual males, and african americans are more at risk. Incubation: It penetrates intact mucus membranes or abraided skin and enters the lymphatics and blood within a few hours, causing metastatic foci long before and after primary lesion appearance. Blood at this time is infectious. Dx: Dark Field Microscopy, with repeat exams. Serologic tests for non-specific reaginic antibody and specific antitreponemal antibody. The former gives false + 20-40%, the latter 1-2% of the time. Most Labs use VRDL (macroscopic flocculation) as a syphilis test, with RPR or ART modifications. This has a 40% false + so treponemal tests like hemaagglutinin and immunofluorescence confirm. false +'ves can be cause dyb viral disease, IVDR, recent imunization, age, and autoimmune disorders (lupus, Rhumatoid arthritis) Dx: Penicillin is the first line, followed by tetracycline/doxycycline, and then azithromycin. During Pregnancy only PCN is indicated. Re-treatment shond be considered when clinical signs/syptoms persist/recur, a 4 fold increase in titer, or failure to achieve a 4 fold decrease after a year (both eval using nontreponemal test. HIV complicates the issue and requires more rigerous and prolonged tx. |
|
|
Syphilis Manifestations
|
Primary Syphilis:
Median incubation time is 3 weeks. The primary lesion is at the site of inoculation and persists 2-6 weeks. It is a single painless papule which repidly becomes indurated and eroded forming an ulcer. Inguinal lymphadenopathy is b/l and may persist for months. Other conditions that may be mistaken are trauma, syperinfected lesions, genital herpes, chancroid. It may also include painless rash and ulceration with a clean base. Secondary Syphilis: 4-6 weeks later. Appearance of diffuse mucocutaneous lesions (maculopapular, pale red, occationally pustular, 90% of skin is involved, incl. on plams and soles), general non-tender lymphadenopathy. **note that we will never see vesicle or bulla with syphilis. The Palms and soles are often involved, and Flat Warts (broad, moist, ping/Grey/white, infectious). Also fever, weight loss, malaise, annorexia, chancre, H/A and Meningismus (but meningitis itself is rare). Hepatitis nephropathyl arthritis, periostitis, iridocyclitis. Latent Syphilis: Starts when the bug is only detectable by blood test. Dx is serological with normal CSF. Genearlly completely asymptomatic. Early latent syphilis (1st 2 years s/p infevtion) and late latent syphilia (begins 2 years after infection). may never cause clinical symptomogy although they pt is not necessarilly "cured." can be 15-30 years. Late Syphilis: Slow preogressive disease of the CV (aorta) system or CNS. Asymptomatic neurosyphilis CSF abnormalities but with no clinical manifestations. Symptomatic neurosyphilis Risk is 2-3 times greater in caucasian pop and twice as likely in men. After infection, 5-10 years we may see meningovascular syphilis (seizure, stroke). 20 years later we may see general paresis (personality, affect ,reflexts, eyes. Senses, intellect, speech [pneumonic], as well as optic atrophy), and 25-30 years later tabes dorsales (spinal involvement leading to ataxia, impotence, bladder disturbances, peripheral neuropathy, and romberg sign. May include CN 2-7. Cardiovascular syphilis: Limited to large blood vessels supplied by the vasa vasorum causing medial necrosis with destruction of the ascending transverse segments. May cause aortic regurgication, saccular aneurism, and Coronary ostial stentosis. Congenital Syphilis: Can occur at any stage of pregnancy, lesions are possible after the 4th month of pregnancy (the begining of immuen competency). Tx before this point prevent fetal damage. Initial signs include heptatomegaly, skeletal abnormalities, low birth weight, skin lesions, jaundice, pneumonia, etc. The child may present with 2ndary syphilis before 2 years of age. Late stages are non-infectius and resulve into residual stigmata including Hutchinsons teeth, frontal bosses, short maxilla, high palate arch/palatal perforation, sabre skin, mulmerry molars, saddle nose, protuberance of mandible etc. The last 3 called hutchinsons triad). |
|
|
Lyme Disease
|
AKA lyme arthritis, is caused by a spirochete Borrelia burgdorferi via the Ixodes tick. IT can be cultured in Barbo-Sroenner-Kelly’s medium., growing best @ 33 degrees. It is the most commonly diagnosed vector borne disease in the US, being present in all temperate regions of the northern hemisphere.
Pathogenesis: Ixodes has to be attached 36-48 hours to be able to transmit disesase. The nymph stage (2nd year) of the bug feeds on animals infecting them asymptomatically, and then becomes adult and spreads disease. It often begins in the summer with a characteristic skin lesion (erythema chronicum migrans), accompanied often by Headache stiff neck myalgias arthralgias malaise fatigue lymphadenopathy. Later manifestations include cardiac symptoms: ocute-onset high grade atrio-ventricular conduction deficits. neurological symptoms: lymphocytic meningitis cranial neuritis *facial palsy Radiculoneuropathy Rarely encephalomyelitis And recurrent brief bouts of arthritis. Dx: The national surveillance of lyme accepts a case if a pt experiences: Presence of Erythema Chonicum Migrans, OR A person with at least one late manifestation and lab confirmation. Immunoglobulin “bands” are often present even in early phase Dx. In general EIA or ELISA for antibodies are used in the lab, often in combination with western blot.. Culture and isolation are definitive but yield for either tends to be low. Tx: Tx should commense as soon as possible, and generally includes doxycycline (early), amoxicillin (Or Doxy- Late), cefuroxime/ceftiraxone (CV/Neuro). The alternative is macrolides. Doses are increased for late manifestations. |
|
|
Meningitis (general)
|
Most of the CSF is produced by the choroid plexus and flows through the channels and ventricles unil it is reabsorbed. We generally have about 1ml/lb of body weight.
problems with CSF evaluation include: The pt may have normal values and it does not always gram stain and culture well. The pt may have + csf as a result of parameningeal proesses (infections) Pleocytosis following stroke, seizure, syphilis etc. Rare forms include Granulomatous (Tb, sarcoid, fungal), Lyme, Syphilis, Drug induced, carcinomatous. Diseases confused wtih meningitis: Subarachnoid hemorrhage, brain abscess, stroke, seizure disorder. Major pathogens include: Streptococcus pneumoniae Neisseria meningitidis Streptococcus agalactiae (group B) Cryptococcus neoformans Miscellaneous Gram-negative rods Listeria monocytogenes Staphylococci |
|
|
Bacterial Meningitis (general Principals)
|
More common in the Winter/Spring.
Sequene of events: 1. Bacterial Colonization of the nasopharynx 2. Local invasion 3. Bacteremia any bacteremia MAY progress to meningitis) 4. Evasion of host defenses 5. Invasion of choroid plexus (most start here and go to the ventricles and then all over) 6. Local growth (in csf) and inflammation 7. Exceptions to bacteremia step a. Post neurosurgery b. Prior Fx of the cribiform plat allows organisms to enter directly via the nose. Properties important for bacteria- Adhesion to the pharyngeal mucosa IgA protease helps sustain colonization Invasiveness Evade host defenses (capsule alternate complement evasion, esp in young kids). Ability to invade CNS Host predisposition: Most hosts normal No immunity to bacterial strain (Immature immunity, no prior exposure, Senescent immunity, concomitant immune disease) Poorly able to clear infection from bloodstream, e.g. absent spleen Rarely drug or cancer induced meningitis is observed. Inflammatory response: Breakdown of the blood brain barrier due to cell wall and membrane components mediated by IL1, 6, and TNF. Alteration of CSF flow Increase in intracranial pressure (may lead to brain herniation) Increases permeation of WBC (continue inflamation) Tx Guidelines: Use High dose antibiotcs as the BBB needs to be breached (although it is somewhat compromised by the inflamatin for the moment). Sustain the high doses and give steroids before and during tx to decrease morbidity/mortality (1/3 of deaths are due to brain herniation). It is vital to start therapy ASAP (within an hour if possible). Steroids ar eonly helpful if given before or with first dose of antibiotics. If we know the bug treat it, but if we are not sure... Check gram stain, if -, Give 3rd gen cephalosporin + vanco to otherwise healthy outpatients and send CX (stop one after cultures grow) If decreased cell mediated immunity of age >50, Add high dose ampicillin for listeria. |
|
|
Bacterial Meningitis (clinical)
|
Clinical Manifestations:
Headache – severe (worst ever) Fever Neck stiffness even on passive motion. Global decline in mental function Relatively quick onset Variably Rash (petechiae)- range of severity, may lead to skin necrosis and circ to hands and feet may be impaired causing necrosis. Hypotension (dangerously low BP) Epidemiology: There is a strong correlation with age: Infants at highest risk Group B strep (S. agalactiae) E. coli Enterococcus sp. Young children and adolescents N. meningitidis or S. pneumoniae Adults S. pneumoniae or N. meningitidis Steroids Listeria monocytogenes Note that H. Influenzae is not a problem now due to the vacine. Labs: Blood- WBC elevated with neutrophils in early forms (differential). BCX + 1/2 of the time. CSF- "pyogenic" pattern in 90% with gram stain + > 1/2. <5% have bland CSF. Normal CSF: 0 red/1-5 white cells <2 neutrophils Glucose > 45mg/dl(about 2/3 of normal serum) Protein <55mg/dl Pyogenic pattern = WBC >100 (often in the 1000's) with a predominance of neutrophils, Glucose <45, Protein >100 (nonspecific). 1/2 of the time Gram stain is +. |
|
|
Viral Meningitis (general Principals)
|
More common in the summer (Aug-Oct). The patients do not look quite as ill and may have a fluctuating mental state. It may cause a rash and often resolves on its own with no serious long term sequellae. Shunts in the head can lead to infection.
CSF: WBC elevated <100 with predominance of lymphocytes. Glucose is about normal, protein is elevated but <100. Gram stain is - vultures are sometimes + from CSF or body sites. |
|
|
Strep Pneumoniae Meningitis
|
Most common cause in adults
Kids – usually lack antibody Adults – often have diminished immune function as a result of Splenectomy. AIDS, Alcohol. Has relatively high mortality rate, but is not epidemic. Tx: It may be resistant to Pcn and Cephalosporins but that is decreasing (really). Vanco with PCN/Ceph until susceptability results are back. A conjugate vaccine in kids reduced it in kids, parents, and grandparents, while the polysaccharide vaccien for adults was not impressively helpful. 1st line tx is ceftriaxone, if it is resistane use Vanco. |
|
|
Neisseria Meningitis
|
Most common in kids and adolescents. Capsular types a, b, c, w-135, y, with vaccines all but type b (the conjugate vaccine is more potent and expensive). Immunity lasts until about 8 yrs old. This is the only one in which person-person transmission is common.
Those infected usually have normal immunity. Can be overwhelming infection that comes on in hours, but lower mortality rate than pneumococcus. Epidemics do happen, but are rare in the US. Characterized by rash, hypotension, necrosis (sequellae of amputation in survivors). Tx: Propylaxis for those in close contact including select health care providers in close contact with secretions. The vaccine is partly effective for most serotypes but takes a few weeks to kick in. First line Tx is Pcn or a 3rd gen cephalosporin. |
|
|
Strep agalactiae Meningitis
|
Pathogen only in newborns
Acquired from mom’s genitals Little risk after 2nd month of life |
|
|
Listeria Monocytogenes Meningitis
|
Food-borne pathogen
Decreased cell-mediated immunity, e.g. cancer, steroids, AIDS May cause brain infections/abscesses at the same time Usually accompanied by bacteremia Preceded by diarrheal disease in some people Tx: Ampicillin or TMP/SMX |
|
|
Gram - Rods and Staph Aureus Meningitis
|
Usually nosocomial
May follow brain surgery May result from bacteremia High mortality (patients are already quite ill, so it is not just the meningitis per se.) Tx: Generally wtih Vanco...dependson the culprit. |
|
|
Cryptococus Neoformans Meningitis
|
Dx:
Cryptococcal antigen test (CSF) Patients have an underlying immune problem/disease. In AIDS patients the CSF is usually normal. Tx: Amphotericin B Fluconazole |
|
|
Respiratoy Tract Infections (general principals)
|
Indigenous microflora form a mutualistic relatinship and are essential for the maintenance of health (Colonizatin resistance). We get colonized if organisims that are not part of the normal brood take up residence. Often they just sit around and do not cause trouble or disease (colonization).
Infection is the result of an organism producing an inflammatory or histopathologic response. The resulting disease may be clinical or subclinical (no signs/symptoms, e.g. primary TB). Mode of Entry: Via blood Suspended in inhaled air (droplet nuclei may be we ot may have dried out but persist in viability). Direct spread from adjascent focus Aspiration from upper airway (the source of most bacterial pneumonias). Aspiration: Obstructive aspiration predisposes to infection as it inhibits clearance. Association of aspiration with alcohol abuse is common. Normally the lung should be starile and the throat colonized. Actually we aspirate all the time, but any depressed consciousness (like when drunk) exacerbates the situation. There are 2 normal defenses to aspirated material: 1. Aerodynamics- particles > 10 microns get stuck on mucus membranes on the way in Particles 2-10 microns get in the airway but as it takes an abrupt turn they get stuck particles 0.5-2 microns can get al lthe way in where we have 2. local clearance mucocilia lymphatic drainage surfactant antibacterial agents professional phagocytic cells (alveolar macrophages) we have problems when these defenses get attenuated (when we are drunk our alveolar macrophages are drunk too, the FLU is the best at this and predisposes to superinfection), or with particularly virulent bugs, or if the volume of aspiration is too high. Generally the sicker you are the faster colonization occurs. In the hospital it is expected that you will be colonized, so that finding bugs in the sputum does not necessarilly mean that that bug is causing disease. Pneumonia in adults: Community-acquired: Pneumococcus 60-80% H. influenzae 10-15% Anaerobes 5-15% Staph. aureus 2-6% Gram-neg. rods 2-5% Hospital-acquired: Gram-neg. rods 75-85% Staph. aureus 10-20% |
|
|
Tuburculosis
|
Still an immense problem, with new populations at risk (AIDS, Transplants). Causes 3 million deaths per year. In the late 80's rates started to rise again after a decline as a result of AIDS, collapse of the med infrastructure, and an influx of immigrants from places with endemic TB. A growing %age of US TB cases are foreign born.
Transmission: Droplet nuclei are inhaled, and end up preferentially in the lower lung (ventilation is higher there). Once there they mutiply and spread to the hylar lymph nodes, and from there into the blood. Incubation takes 4-5 weeks during which we have the onset of cell mediate immunity and granuloma formation. At this point a person who had a - ppd now is +, used to document primary infection (5% sick in the next 5 years, 5% sometime in life, 10%/year in AIDS). Some may remain viable inthe granuloma for decades (if it becomes calcified they die). This primary infectin is often but not 100% asymptomatic. Then when the patients immune system is supressed the granulomae can break down and lead to a reactivation response. The lesions cavitate on X-ray, and may be b/l. They also will show miliary spread when they get into the blood (tiny nodules in the periphery of the lung (look like millet seeds, indicates massive infection). Determinants of infectivity: Is the donor coughing/sneezing Is the environment closed or open (density of infectious particles) Host factors (state of health, AIDS, etc.) PPD: If it is + you have latent TB infection (asymptomatic), but there is the potential for activation. We give INH to prevent activation and disease (INH prophylaxis AKA tx of latent TB infection, highly effective). |
|
|
Endocarditis (general)
|
Defined as amicrobial infeciton of heart valves or endocardium.
Subacute bacterial endocarditis (SBE): Insidious onset Symptoms prior to Dx: weeks-months Preexisting damaged valve viridans streptococci Acute bacterial endocarditis: Acute onset- less than 5 weeks Normal valve often Staphylococcus aureus Pathogenesis: Hemodynamic Change- anatomical alteration results in differences in blood flow pattern (high pressure zone to low pressre sink) as in mitral Regurgitation (Alos VSD, but not ASD). Platet-Fibrin thrombi- “Vegetations” made up of sterile platelet-fibrin thrombi form when hemodynamic change causes trauma to the endothelial surface and exposes collagen. Bacteremia- Bacteria need to enter the bloodstream to gain access to the valves. This can happen more frequently than we might think...Dental work, brushing teeth, etc. Lots of bugs get in, including 3 kinds of strep. Adherence- the bugs have to stick to the valves/vegetations. Anti-stick meds are coming in the future. Ex. We have lots of E. Coli bacteremia, however it does not adhere well and so rarely causes endocarditis. Heart disease predisposes to endocarditis including: Mitral prolapse with regurg Theumatic fever Atherosclerosis Congenital malformation prosthetic valves (especially prone) A-V shunts Trauma from central line Previous endocarditis Bacteriology: Staphylococci (most aureus) (30-40% (esp for IVDR, S. Epidermidis most common for almost all foreign body placements like valves)) Streptococci (most viridans) (20-30%) Enterococci (10%) Gram-negative rods (2%) Fungi (2%) Culture negative (8%) (Prior antimibrobial therapy make the bacteremia/bugs tough to detect clinically. the most common cause is either bartonella or mycobacteria. |
|
|
Endocarditis (clinical)
|
Infection Symptoms:
Fever Chills, sweats Leukocytosis (incr WBC count) Elevated sedimentation rate/CRP (nonspecific markers of inflamation) Anemia splenomegaly pleuritic chest pain Cardiac involvement/symptoms: Murmur (hallmark of endocarditis) Heart failure (mjr cause of death in endocarditis) Heart block (with electrical involvement) The resultant emboli cause: Stroke Skin lesions Hematuria Metastatic infections Spleen infarction/abscess due to infected emboli (mycotic aneurysm- muchroom shaped, embolism to the vaso vasorum) Cavitary lung nodules (tricuspid valve) Resultant immune complexes cause: Arthritis Glomerulonephritis (hematuria, not caused by infarct here) Vasculitis petechiae, splinter hemorrhages, Roth spot (heomrrhage on the retina with a central white spot), Osler’s node (swollen tender finger/toe pulp, many splinter hemorrhages), janeway lesions (flat non-tender lesions) Rheumatoid factor Decreased complement Consider endocarditis when: Fever and a murmur Fever and injection drug use Prolonged Fever of unexplained origin (FUO) Fever and systemic emboli (stroke) Fever after valve surgery Dx: Clinical picture (presence of peripheral sequellae) Blood Cultures (generally times 2 from seperate sites to eliminate transient bacteremias) Echocardiogram (visualizes valves) Duke Criteria Tx: Bactericidal drugs (here is one case where the difference in important…also in meningitis). Synergy between meds is sometimes used, high doses over a long time. Note that the bugs divide mroe slowly in the vegitations (every 2-3 days) so if we are using cell wall active meds we need them over the long term. Indications for Surgery: Native valve- intractable heart failure fungal endocarditis persistent bactermia myococardial abscess giant vegetations? Pseudomonas? Prosthetic valve- most require surgery as the infection causes the valve to separate from the heart wall. Prophylaxis Give antibiotics to those at high risk @ the time of procedures that produce bacteremia. selection is directed at common offenders, and is the standard of care, but there is no proof of effectiveness, and most endocarditis is related to every day bacteremia rather then procedure oriented. |

