![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
103 Cards in this Set
- Front
- Back
|
Gram + Bacteria
|
staphylococci aureus
epidermidis saprophyticus Streptococci group A-T pyogenes agalactiae faecalis (Enterococii, Group D) pneumonia (pneumococci) Corynebacteria Diptheriae jeikeium (JK) Listeria monocytogenes Erysipelothrix rhusiopathiae |
|
|
Staphylococci Aureus
|
Gram + cocci form grape-like clusters or appear as singles. In vitro they form creamy yellow (or white) pigmented colonies. They produce coagulase and B-hemolysis. Catalase +, halotolerant, primarily aerobic, and most ferment mannitol. Phage typing is an important epidemiological tool.
Produces: Protein A - bind IgG, antiphagocytic Capsule- antiphagocytic Coagulase - fibrin formation around bacteria, antiphagocytic. May facilitate localization/abscess formation. Catalase - H202 Protection, diagnositc. Staphylokinase (fibrinolysin)- allows clots to release organisms allowing systemic dissemination. nucleases and proteases - obscure relation to pathogenicity lipases - degrades fats and oils, facilitates colonization of sebacious glands. Hemolysin - 4 kinds that destroy RBC's, neutrophils, macrophages, and platelets. Alpha type is dermonecrotic, hemolytic, and lethal. Leukocidin - destroy WBC's Penicillinase - PCN resistance Novel PBP-2 - methicillin resistance (MRSA) Hyaluronidase - Spreading factor, breaks down conective tissue. Exfoliatin - diffusible exotoxin causing Scalded Skin Syndrome (SSS) Enterototins - Heat stabile caue of food poisoning Pyrogenic exotoxin (Toxic shock syndrome Toxin TSST1)- causes TSS by acting as a superantigen, massively activating T cells releasing TNF and IL. In general it forms abscesses for local infestation, but can spread via lymphatics/blood. Some are normal flors and infect opportunisticaly. Staphylococcal enterocolitis - invasion with pathogenic staph colonizing the bowel Staphylococcal food poisoning - intoxication due to ingestion of enterotoxin. heat kills bugs, but toxin is stabile. Stimulates brain/enteric NS to cause vomiting and diarrhea. Toxic Shock Syndrome - Impregnated tampons/cotton paking left in too long can cause release of TSST1. Causes vomiting, diarrhea, desquamation of palms and soles, diffuse red rash, fever, septic shock. Scalded Skin Syndrome - caused by exfoliatin toxin dessiminated to the skin. infects cut imbilicus of neonates and children with skin infections. Looks like water burn abuse, drug allergy. Can cause boiles or feruncles, which can convalesce to form carbuncles. delayed hypersensitivity results in inflamation causing >50% of the discomfort. Pneumonia, meningitis, osteomyelitis, paranicia (in nails from biting), acute bacterial endocarditis (as opposed to SBE, esp with IV drug abuse),septic arthritis, skin infections (impertigo), bacteremia/sepsis, UTI Cellular immunity is more important for killing bacteria, but humeral is more important for anti-toxin effects. |
|
|
Streptococcus Pyogenes
|
Gram + Group A Beta Hemolytic Cocci forming chains and producing small glistening colonies. Morphology is based on the antiphagocytic hyaluronic acid capsule. Lactic acid fermenters producing acidic environments (self limiting). Catalase -, bacitracin sensitive (diagnostic). They produce a number of antigens :
C Carbohydrates – in the cell wall, used for lancefield typing, antigenic M protein – in the cell wall and forms antiphagocytic pilli, covered with LTA, the major virulence factor which inhibits complement and is antiphagocytic. Non-Antigenic and susceptible to Ab. Streptolysin O – Enzyme, O = oxygen labile, destroys erythrocytes (responsible for B hemolysis), antigenic (Ab’s develop following infection and is used diagnostically. Role in Sequellae? Can break clots and spread infection. Streptolysin S- Enzyme, S = oxygen stabile, B-hemolytic, non antigenic. (can produce either depending on O2 status), can break clots and spread infection Pytogenic Exotoxin – (AKA erythrogenic toxin, lysogenic conversion) exotoxin responsible for scarlet fever, not in all strains. Red rash that spares the face. Toxin breaks endothelia of capillaries. Infections Processes: Streptococcal Pharengitis – swollen tonsils and pharynx, pus, fever, Dx via rapid strep followed by culture. Scarlet Fever – Pyogenic exotoxin causes fever and red rash on neck and trunk moving to the extremeties but sparing the face. Necrotizing faciitis – “Flesh Eating,” enter via a break in skin and move along fascia, swelling, heat, redness, eventually necrosis. Wound/Burn bacteremia – opportunistic? Child-Bed fever – purpural fever in places with poor asceptic technique. Toxic Shock Syndrome- pyrogenic toxin causes fever, hypovolemia, rash, etc. Likely superantigen mediated Endocarditis Post Infection Sequellae: Rheumatic Fever – S/P pherengitis, Fever, Myoarditis, Arthritis, Chorea, Sub-Q nodules, Rash. Can build after repeated infections over many years. Acute glomerularnephritis – s/p pharengitis or epidermitis by nephritogenic strains. Ab/Ag complexes deposit in glomerulae, activate complement, and destroy. Tea colored urine/edema/hypervolemia. Approx 1 week post infection Therapy: Susceptable to penicillin, but less so today than before so we often use penicillinase resistant PCN. Also Cyclosporin as it halts exotoxin synthesis. |
|
|
Streptococcus Agalactiae
|
Gram + Group B Beta Hemolytic Cocci. Catalase -. Members of the normal vaginal flora, can cause fetal pneumonia, sepsis, and meningitis via innoculation in the birth canal. can also infect Mom causing bacteremia and sepsis. Dx Via lumbar puncture and Tx for Group B strep, E. Coli, and Listeria Monocytogenes. Also, Culture pregnant women and give prophylactic antibiotics as bith approaches.
|
|
|
Viridans Streptococcus
|
alpha hemolytic (Green = viridans) cocci. Part of the normal flora found extensively in the teeth and mouth.
Subacute Bacterial Endocarditis (SBE)- Dental work causes these bugs to flood the blood stream. They produce a dextrose that allows them to adhere to previously damaged cardiac valves (eg. from rhumatic fever). They slowly grow (hence subacute) causing fever, fatigue, anemia, and heart murmurs. Note that this can also be caused by G-hemolytic strep including some group D. |
|
|
Group D Streptococcus
|
(AKA Enterococcua faecalis or Enterococci) Gram + cocci, alpha, beta, or gamma hemolytic. Enterococci (E. Faecalis and E. Faecium) are halotolerant (6.5% NaCl), and PYR+; also bile tolerant and hydrolyze esculin. Non-Enterococci Group D (S. bovis) Strep are non-halo tolerant, PYR-, Bile tolerant and Esc+ (used to screen for all Group D).
Enterococci are part of the normal bowel flora and often cause UTI's, Biliary Tract Infections, and SBE as well as dirty wound/soft tissue infections. They are more resistant to PCN that other strep, as well as aminoglycosides. Now Vanco resistant strains (VRE) have been found that can txfer DNA to others. |
|
|
Streptococcus Pneumoniae
|
(AKA Pneumococci, Diplococci?). Gram + lancet shaped diplocicci (or in chains). Alpha hemolytic, cat -, fastidious, shiny colonies when pathogenic. Identified on the basis is the quelling reaction (exposure to antiserum causes cell wall to bulge), bile sensitivity, and the optochin test (P-Disk with optochin causes zone of clearing). The most pathogenic strains are type 3, 6, 14, 19, and 23; pathogenicity being based onthe the amount of antiphagocytic polysaccharide-capsular material present (toxin production is not related, and acapsular pahtogenicity is opportunistic only). This is also the basis for antigenicity. It also produces Secretory IgA protease to allow colonization and Pneumolysin to injure epithelial cilia.
Found in normal throat flora (many healthy carriers), it is the most common cause of pneumonia and mengitis in adults, and otis media in children. Also causes sinusitis and bronchitis. Note that Iv Drug use/Alcohol use are risk factors? Tx with PCN is now only marginally effective, and other resistances are developing. Newer prophylaxis includes vaccines: pneumobax contains 23 antigens for Pneumococci and is indicated in children and immunocompromised pt's etc. |
|
|
Staphylococcus Epidermidis
|
Gram + cocci producing white colonies. G-hemolytic, Coag -, catalase +, facultative.
Generally nonpathogenic (normal skin flora) but can be opportunistic. Less antiphagocytic than S. Aureus. Common cause of nosocomial infections (can originate from the pt [endogenous] or elsewhere[exogenous, healthcare worker-iatrogeic]. Produces a biofilm/slime that allows it to adhere to and migrate along abiological devices like Iv's/ Foley's etc...allowing opportunistic infection. A common contaminant of blood draws for lab eval. The most common bacterial isolate of indwellind prothethic devices as well (hips, heart valves, etc...), also effecting wounds, immunocompormised pt's, surgeries, etc. Most are PCN resistant and some are even MRSE's. Also the biofilm gives them intrinsic protection. |
|
|
Staphylococcus Saprophyticus
|
Gram + micrococci forming white colonies. Coag -. Urinary pathogen (UTI) in young sexually active women. Differentiated from S. Epi by its resistance to novibiocin (Dx, not Tx). Can be on the skin in "interternchenous" areas. Most commonly community aquired.
|
|
|
Gram Negative Pyogens (list)
|
Neisseria Meningitidis
Gonorrhoeae Sicca Flavescens Branhamella Catarrhalis (Moraxella) Heimophilus Influenzae parainfluenzae Ducreyi Bordatella Pertussis Parapertusis Pseudomonas Aeruginosa |
|
|
Corynebacterium Diphtheriae
|
Gram + club shaped rods/pleiomorphic. Grow well on most common media, differentiated by tellurate selective media and biochemical tests. Non-pathogenic until a temperate (lysogenic) phage confers diphtheria toxin.
Disease presents as a localized pharyngeal upper respiratory infection producing a grey/white membrane/cast consisting of fibrin, inflammatory cells, debris, and bacteria. Morbidity and mortality result from pharangeal obstruction, and toxin dissemination. The Toxin itself is a B/A complex causing ADP ribosylation of EF2, thus inhibiting protein synthesis. It is most devistating in tissues where protein synthesis is important (heart, diaphragm, brain). Tx includes antitoxin, antibiotics (most will work), and a vaccine/booster (although this will not be immediately effective) |
|
|
Corynebacterium Jeikeium
|
(AKA "JK," Coryneform bacteria, diptheroid) Gram + rod/pleiomorphic. JK is a diptheroid, associated with bacteremisa in patients in intravascular catheters, surgical wounds, and prosthetic heart valves,(native valve endocarditis as well) or other implants, esp opportunistic in immunocompromised pt's, nosocomial infection. There are many diptheroids that are similar, JK being a prototype. Should be grown on special media and confirmed by biochemical tests. Note that this is part of our normal flora and inhabits our skin, mucus membranes, water, and soil, so they are frequently listed as contaminates to our sample. Highly resistant to most common antibiotics, use Vancomycin.
|
|
|
Listeria Monocytogenes
|
Gram + rod, facultative. Non spore forming. 1-5 flagella, grows well at 25 Degrees (often cultured at 4-10 degrees to differentiate it (cold enrichment).
Major virulence factor listeriolysin O allows it to escape the phagolysosome/death and become a facultative intracellular organism. Cell immunity is important therefore, and the immunocompetant can fight the infection off. Pregnant Women, Neonates, the elderly, and the immunocompromised are at risk. The pregnant woman is vulnerable when cellmediated immunity is low in the 3rd trimester, and the neonate is vulnerable from her. A leading cause of meningitis in newborns (confirmed by high protein, low glucose gram stained CSF), which is treated by TMP/Sulfa or ampicillin. Other then person to person, listeria can be aquired by ingestion of contaminated coleslae, milk, soft cheeses, butter, and deli meats (so late pregos should avoid them) |
|
|
Erysipelothrix rhusiopathiae
|
Gram + rod.
|
|
|
Neisseria Meningitidis
|
Gram - diplococci (opposed kidney beans/gram - donut) that grow best with increased CO2. They are fastidious and grow on chocolate agar. Thayer-Martin VCN media selectes for them by including Vancomycin (kills gram +), Colistin (polymixin kills other gram -), and Nystatin (kills fungi) to eliminate flora. Maltose Fermentation +.
Virulence Factors include: Pilli- for initial attachment to nasal epithelium IgA protease- aids in colonization/protection Outer membrane proteins- bind IgG backwards to block antibodies Lipooligosaccharide (endotoxin)- shed in blebs, causes endotozemia and thromboembolytic lesions. normally 4-10 % of healthy people are carrier, up to 30-90% during and epidemic. Most adults have bacteriocidal antibodies, and neonates are passivle immune, but kids from 6 months to 2 years are esp at risk, as are new college dorm freshmen and military recruits. Also those with complement deficiency or asplenic individuals. causes upper respiratoty symptoms and fecer, to miningococcemia with high fever, weakness, petchial rash (halmark of Dx), to Meningitis (3 common causes are HIB, N. Men, and Strep Pneumoniae), Endocarditis, and waterhouse-Frederichsen syndrome (adrenocortical necrosis) There is a vaccine contains antigens for serogroupa A, C, Y, and W135 (B is not immunogenic), and is used mostly in the military and for international travelers. Infection is treated with massive penicillin (ineffective against carriers) doses, Prophylaxis is via rifampin, cipro, or ceftriaxone |
|
|
Neisseria Gonorrhea
|
Gram - diplococci, Gram - donuts. Catalase +. No serological tests, require chocolate agar (with antibiotics to diff from normal flora when sampling anogenital area, W/O for blood, joint fluid, eyea, less flora). Delicate organism requires culture ASAP. Suspect colonies are subjected to sugar utilization tests, slide agglutination (esp oropharynx, eyes, non anal). non-culture tests preserve specimens for transport (gram staining etc) and Elisa, Genitic transformation tests, and nucleic acid amplification tests (NAAT) are available.
In Women, culture endocervix and anus to check for carrier status. No pre-culture tx unless she is the partner of a known carrier as she has gram - diplococci flora. In Men urine specimen is viable, urethral exudate or anterior urethral swab(less flora as well). Throat cultures form those who engage in oral/genital sex. Incubates in 2-7 days (14 max) infecting columnar and transitional epithelium (endocervix, male/female urethral and anal canal. Likely concurrtne infection with chlamydia (tx for both)and are more susceptable to other STD's. One can not aquire from toilet seats, requires exchange of body fluids. Ping-pong effect when one partner is asymptomatic and the other cured, passes back and forth leading to repeated infections (not failed tx). Pathogenicity/Virulence- Pili and OMP- attach to epithelial cells (even non-ciliated, preferentially columnar) Parasite directed endocytosis- into non-phagocytic cells (unique) LOS/Sialic acid- Interferes with complement, also endotoxic Catalase- allows intracellular survival. Genetic rearrangements of pilli and opa (makes colonies opaque)- delay effective antibody response. In Males- urethral infections can subside without tx, but sequelae (urethral strictures, epididymitis, protsttitis) are possible with repeated infections. That is rare is th einfection causes pain on urination and purulent pus so most men seek tx. Females- Many cases are asymptomatic leading to higher incidence of sequelae including fallopian tube stricture/inflamation/scarring, pelvic pain, ectopic pregnancy, recurring pevic inflamatory disease (PID). These can lead to Dissemiated Gonococcal infections (DGI) resulting in arthritis, tenosynovitis, skin lesions (wrist, elbows, ankles), pnoncoccal peri-hepatitis (Fitz-Hugh-Curtis syndrome), sterility, and meningitis. Oral sex leads to pharyngitis, and anal sex to rectal gonococcal infection. Children born to infected women may develop Opthalamia neonatorum, which may lead to blindness and is tx prophylactically in all neonates. Sexual abuse leads to vulvovaginitis as a result of increased vginal pH. There is no lasting immunity due to genetic variation and no vaccine. Tx is by fluroquinolones and combo's. Always Tx for gonorrhea and chlymadia. Epidemiologic Tx is desirable (Antibiotics when Dx is likely based on behavior, clinical/epidemiologic grounds before conformation (esp partners of carriers). Condoms and Diaphragms are protective as are prophylactic antibiotics. |
|
|
Neisseris Sicca, Neisseria Falvescens
|
Gram -, Common in normal pharynx, can ocasionally cause infections
|
|
|
Branhamella (Moraxella) catarrhalis
|
Gram -, common commensal in the throt, causes otitis medis in children (less common then S. pneumoniae or H. influnza. May cause other infectiosn in severely ill/immunocompromised patients (upper respiratory infection in COPD [either non-typable H.I. or Branhamella]/Elderly pt's).
|
|
|
Haemophilus Influenzae
|
Gram - rod. Culture on chocolate agar or other media enriched with factor X (Hemin) and V (NAD) both of which are necessary for growth. High CO2 is also required for some strains. X and V tests are used for Identification, and antibiotic sensitivity tests are indicated. Biotyping (I - III) is possible but not usually done. Gram staining, and slide agglutination tests to shed antigen are useful.
In general the capsulated strains cause the disease (typed A-F), but not always. Primarily opportunistic endogenous infection, children are at risk though as it may be carried in the anterior nares/upper respiratory system. Virulence factors: Pili - surface adhesion attaches to nasopharynx IgA protease - facilitates colonization/protection Capsules - antiphagocytic poysaccharides of 6 kinds (a-f). B caused 95% of infection before HIB vaccine. Endotoxin - present but not prominent feature. Pre-vaccine serotype B was the most common cause of disease, however the HIB vaccine has changed that. HI mostly affects young children, but older kids and adults are also suseptible. Diseases include Upper respiratory tract infections- bacteremia- septic arthritis- Most common cause of septic arthritis in infants. Commonly a single jiont affeted, fever, pain, swelling, decreased mobility. Draw fluid for gram staining (Gram - pleiomorphic rods) osteomyelitis- sepsis- pericarditis- epiglottitis- HIB causes rapid swelling of epiglottis obstructing the airway/esophagus. Develops as sore throat and fever followed with seere upper airway wheezing(stridor), inability to swallow, drooling etc. Epiglottis looks like a red cherry at the base of the tongue, manipulation may cause laryngospasm. Establish an airway (Traheostomy if necessary) and tx wtih Ab's meningitis- Infants do not present calssically, but with fever, vomiting, and change in MS. In kids had 3-5% mortality, many had hearing loss or mental retardation. Tx meningitis with Steroids (to attenuate response to lyced bacteria) and then Ab's even before Dx and then streamline (start /w chloramphenicol and Cephalosporins, if susceptible to ampicillin make a switch). Otitis media- often caued by non-typeable (no capsule) HI (2nd most common behind strep pneumoniae) Brazilian purpuric fever- Unencapsulated HI (biotype III; HIB = I), found in brazil, started wtih purulent conjunctivitis, progressed to acute fever, vomiting, abd pain, petechiae, purpura, vascular collapse, shock and Death within 48 hours. Infants get passive immunity, and adults have aquired immunity (opsonic Ab and bacteriocidal Ab with complement), but geriatric pt's are at risk. HIB vaccine is Polyribosylribirol phosphate capsule (PRP) covalently linked with a T-dependant antigen. Decreases both carriers and infections. Household and daycare contacts are at risk, Prophylaxis is rifampin |
|
|
Haemophilus Parainflunzae
|
Gram - Rod. Part of normal flora, rarely cause disease but common in upper respiratry infections. Differentiate from HI using X and V factor, (it only requires V)
|
|
|
Haemophilus Ducreyi
|
Gram - rod causes Chanroid (STD). Common in tropical climates. Causes soft chancre, genital suppurative ulcers which are painful with a unilateral painful swollen inguinal lymph node. Also buboes- 2ndary lesion due to contact with the primary (kissing lesions). We need to diff from Syphilis (Treponems Pallidum) which causes non-painful ulcers, Herpes which causes paunful ulcers with systemic symptoms (myalgia, fever), and Lymphogranuloma venerum (LGV-Chlamydia trachomatis) which has painless lymph nodes that do not swell until the ulcer heals. The open sore increases risk for HIV transmission. Dx made on clinical pic and elimination of other STD's. Gram staining of aspirates is possible, culture growth is poor (chocholate agar works occasionally)
|
|
|
Bordatella Pertussis
|
Gram - Rod, only grow on special media (Bordet-Gengou medium with Ab's to inhibit normal gram + flora). ID is made via ELISA, PCR, fluorescence, slide agglytination, no biochemical tests as it is biochemically inert. Originally in kids, now about 50% in adults as the vaccine only works for about 15 years. The vaccine itself was originally DTP (whole cell components) but today is DTaP (acellular pertussis). As passive immunity in adults is not strong, infants are at risk. This is in the top 10 ID causes of death worldwide.
Spreading by droplet nuclei during the initial stage of infection (when you need to take a culture). This is a non-invasive culture, binding to ciliated epithelial cells and releases toxins from there. Pre-immunization babies and young adults (vaccine only lasts 15 years) are susceptible. Virulence: Filamentous hemagglutinin- aids in attachment to cilia Tracheal cytotoxin- injures respiratory epithelium cilia Pertussis Toxin - (AKA lymphocyte promoting factor)- promotes lymphocytosis and likely enhances attachment to epithelium. A-B toxin, causes ADP ribosylation regulatory proteins for Adenylate cyclase, increasing fxn. Extra cytoplasmic adenylate cyclase- when attaching bronchi BP releases adenylate cyclase which is taken up by lymphocytes, macrophages, and and neutrophils, increasing cAMP and inhibiting chemotaxins and ROS production. It is activated by host calmodulin and other hemaglutinins. These virulence factors are controlled genetically in a cascade of events based on temp and ionic environment. Hemaglutinin and Pili comes on first, and then Toxin and Adenylyl cyclase production. Disease: 3 stages... Cattharal stage lasts from 1-2 weeks, high bacterial load, the time of highest level of contagion when cultures have to be taken. resembels upper erespiratory infection (cough, rhinitis, sneezing, low-grade fever). As this bug will not grow on cotton, we often have the pt cough onto media. Paroxysmal stage: The fever subsides and the pt has bursts of non-productive coughing. They are violent and the inspiration through the narrowed glottis causes the "whoop" (may be neurological in nature). Can last for a month of longer, and has a range of severities (most severe in kids, and the less severe formin adults may be misdiagnosed at times). Most deaths in the US from BP are from 2ndary infections (Strep pneumoniae for instance) convalescent stage: attacks grow less frequent over a month and the pt is no longer contagious To tx we immunize, antibiotic tx is important in the catarrhal stage but from the paroxysmal stage onward they are of little value. Erythromycin is preventitive |
|
|
Bordatella Parapertussis
|
Gram - rod. Not Common, Cause of a mild whooping cough
|
|
|
Pseudomonas Auerginosa
|
Gram - rod, obligate aerobe. Produces blue and green pigments and a grape/fruity smell on infected dressings as well as in culture. It is ubiquitous in nature (soil, water, vegetation, and in humans). Note that there is no person to person spread, only environmental. It does not affect healthy individuals, but compromised hosts.
In the lab, it grows on any routine media, often MacConkey's agar which counter selects gram + organisms, and is poor in nutrients but ok for enterics, PA, and non-fermenting bacilli). Pyocin and serotypin tests are possible but not routine. in Cystic fibrosis pt's they characteristical had mucoid colonies due to the capsule. Alginate overproduction is a result of the high osmolarity in the thick lung secretions. Diseases: Pneumonia - most cystic fibrosis pt's are colonized and their lungs are destroyed. Other immunocompromised pt's (cancer, ICU, etc...)are also at risk. osteomyelitis- Diabetic pt's foot ulcers can lead to bone infection, IV drug use (infect vertibrae of clavicle), and children with foot puncture wounds are at risk. Burn/wound infection- the organism can be smelled in burn wards. Can lead to sepsis. Sepsis- high mortality rate sepsis. otitis externa- malignant external ear infection burrows into mastoid bone, particularly in elderly diabetic pt's. swimmers ear/whirlpool skin infections corneal infetions- esp in contact lens wearers UTI/pyelonephritis- in debilitated pt's (nursing homes etc) often having urethral foley catheters as an infection source. Endocarditis- Staph Aureus and PA are freq causes of right heart valve endocarditis in IV drug users Contribute to peritonitis, gangrene. Virulence Factors: Pili- adherence to cells Polysaccharide Slime (alginate) adherence to cells Toxin A- identical mode of action to diptheria toxin (ADP ribosylates EF2, inhibits protein synthesis) but with different antigenicity/binding. Exotoxin S- protease which acts on immunoglobulins and complement Phospholipase- destroys membane of cells Endotoxin Immunity is poorly defined, and there is no vaccine. Tx is tricky as it is resistant to almost every antibiotic we have as a result of outermembrane porind restricgin entry and plasmids. Prevention, be healthy and normal. Avoid catheters of all sorts. watch the hottubs:) |
|
|
Burkholderia Cepacia
|
Gram - Rod, originally classed as pseudomonas. Lung infection in children with cystic fibrosis. very resistant to antibiotics
|
|
|
Burkholderia Pseudomallei
|
Gram - Rod, originally classed as pseudomonas. In the tropics it causes pneumonia, and septicemia. in US soldiers in Vietnam it was called melioidosis
|
|
|
Stenotrophomonas maltophilia
|
Gram - rod, originally classed as pseudomonas. Nosocomial infections of wounds, blood, UTI, and respiratory tract in Cystic Fibrosis pt's and neutropenics (low neutrophils)
|
|
|
Actinobacter
|
Gram - bacilli/coccobacilli. Originally confused with neisseris in urethritis, ubiquitous in nature, causes pneumonia, wound infections, septicemia, and nosocomial infections
|
|
|
Bacterial Pneumonia in AIDS
|
caused by
1. Streptococcus Pneumoniae 2. Haemophilus Influenzae (non-typable) 3. Pseudomonas auerginosa 4. Staph Aureus |
|
|
Infections likely as a result of hemodialysis
|
MRSA
Staph Epidermiditis Pseudomonas Auerginosa Stenotrophomonas Maltophilia Acinetobacter enterococci Fungi Hepatitis Virus |
|
|
Enterobacteriaceae (general principals)
|
Gram negative bacilli
non-spore forming Ferment glucose Facultative Live in the GI tract, and as opportunistic pathogens can penetrate to the blood strem and become systemic. Mote survive in well water or food outside the host. Many are normal flora that help metabolize vitamins and fats. The most abundant is E. Coli (many serotypes defined by O, K, and H antigen combination). Note that normal gut flora also contains aerobes, gra +'ves (S. Aureus), other non enterobacteriaceae gram -'ves (pseudomonas) and fungi. Note that of the normal flora, only E. COli has a strain that produces diarrhea. |
|
|
Vibrio Cholerae
|
Comma shaped gram - rod with a polar flagellum. 2 biotypes agglutinate antigen O-1, the classical and El Tor, both pathogenic. Of the remaining O antigens (2 - 139), only O:139 (bengal strain) is pathogenic. Man is the only natural host, so infection is Fecal-Oral via contaminated water/food. No person to person spread. Some people get mild or no symptomology and are carriers.
Dx is via darkfield microscopy (these guys are small), they can grow on thiosulfate citrate bile sucrose (TCBS) media and are agglutination tests are done (O-1 antigen). Pathogenesis: Polar flagellum- motility TCP (Toxin Co-regulated Pilli)- Adheres to intestinal mucosa and causes activation of toxin production. Note that VC is non-invasive. Choleragen (toxin)- 2 active subunits and 5 B units. The latter ginds to GM1 (ganglioside) and the former activates a Gs protein increasing adenylate cyclase. This increases cAMP which causes the pumping out of NaCl, and inhibits reabsorption. water, potassium, etc follow by osmosis. Toxin productin is controlled by a regulon under control of the "TCP" Diseases: Cholera- Causes voluminous "Rice Water" stools with fluid loss reaching up to 17L/D Therapy involved rehydration by IV or orally with ORS (oral rehydration salts). Tetracycline will lessen the time course of the disease. Infection causes antibodies to form that are vibriocidal, agglutinating, and toxin neutralizing. Immunity lasts for up to 12 months, but note thta Ab to the toxin alone is not protective. Ab to whole organisms prevents adherence, so Serum IgG and IgA are protective, as is gastric acidity. 2 vacines are available but neither are legal in US. (Live VC with deleted A subunit or Killed cells and Added B subunit of toxin). |
|
|
Regulon
|
A group of genes necessary to cause disease in the host. In cholera for instance, the regulun includes the pilus genes, Choleragen gene, and several other minor toxin genes, all under control of 3 activators regulated by the TCP.
|
|
|
Vibrio Parahemolyticus
|
Marine bacteria infectin shellfish. Major cause of food poisoning/diarrhea in Japan, also effects US (mostly on coast in summer) when we eat raw shellfish.
Symptoms - explosive diarrhea (NO blood or mucus), cramps, nausea, fever, vomiting. Produces thermostable hemolysin (kanagawa phenominon), incubates in <24 hours and grows best on TCBS (like VC). |
|
|
Vibrio Vulnificus
|
Marine organisms that infect bathers and those ingesting raw shellfish. Produces fatal bacteremia. The immunocompromised and those with liver disease/iron overload (thalassemia) are most at risk.
|
|
|
Campelobacter Jejuni
|
Curved gram - rod (looks like VC) with a single polar flagellum. #1 cause of bacterial gastroenteritis in the developed world. A Zoonosis with reserviors in cattle and some poultry, transmitted via contaminated water or RAW MILK. High pediatric incidence.
Invades the SMALL intestine causeing severe abdominal pain, fever, headache, and diarrhea with BLOOD and PUS. Identified by the fact that it grows best at 42 degrees in a microaerophilic environment. |
|
|
Helicobacter Pylori
|
(AKA Campelobacter Pylori) Recently identified as a cause of gastritis, and duodenal (leading before ASA) and gastric (Trailing ASA) ulcers. Makes a urease that produces NH3 from urea (pt may have ammonia breath). Tx with an antibiotic cocktail and bismuth salts (in Pepto Bismol which inhibit growth.
|
|
|
Shigella
|
4 species, all pathogens (not normal flora), dysenteriae (most severe), flexneri, boydii, sonnei (last 2 most freq in US). Man is the principal host but monkeys and dogs have been known to be infected. Fecal-Oral transmission via vectors, commonly associated with overcrowding, poor sanitary conditions, nursing homes/homes for the mentally retarded. sonnei is in day-care centers, and flexneri is in gay men.
Virulence: Shigella invade the mucosa of the LARGE intestine and destroy epithelial cells, attract PMN's (purulent), and cause mucus and scar production. The main virulence factor is INVASIVENESS. antigens for invasion are on a large plasmid. It invades one cell and uses actin polymerization to propel itself laterally (non-motile) Shiga Toxin (dysenteriae) is an exotoxin casing fluid exudation. It bahaves like a neurotoxin, 1A-5B units that bind to the 28S subunit of RNA halting protein synthesis and killing the cell. Damage to cells and fluid exudation may contribute to diarrhea, but note that toxin is not as important a virulence factor as invasiveness. Disease: Shigellosis presents with fever, chills, abdominal cramps, tenesmus (ineffective urge to defecate), and stools with BLOOD, PUS, and MUCUS (a result of ulceration of the colon and PMN's?). Milder forms with only watery diarrhea are not uncommon. Dx is via a rectal swab plated on selective and differential media. Note that some strains of sonnei are multiply antibiotic resistant due to an plasmid with resistance genes attached to a "resistance transfer factor." Previous infection gives only short lies immunity and there are only experimental vaccines at present. |
|
|
Salmonella Enteritidis/Typhimurium
|
(AKA salmonella enterica serotype typhimurium) Lactose NON-fermenting Pathogen, not part of normal flora. Any of the 1700 salmonella speies can cause salmonella gastroenteritis (food poisoning) with the exception of S. Typhi. A Zoonosis (communicable form lower animals to man under natural conditions), we commonly ingest food from infected animals including eggs, poultry, pork, RAW MILK, and beef. Food may be contaminated during processing or preparation. Direct transmission from animals via fecal oral route with vectors is also possible, as well as from infected people (food handlers)
Virulence- surface adhesion and invasion of mucosal/SMALL intestine epithelial cells. A toxin may mediate fluid excretion. Tissue architecture is disrupted. Disease - Food poisoning onset is 24-28 hrs causing diarrhea, fever, cramping, nausea, voniing, headache. Symptoms can range from mild to cholera-like. Dx is via fecal culture. Lactose NON-fermentation, slide agglutination, classification of type A-E (depending on O antigen). Within a group , a species is differentiated based on the flagellar and Vi antigens. There is no vaccine, Tx is via fluroquinolones (but studies are showing prolonged excretion of pathogens after antibiotic tx, but newer ones do not agree, and show shortening duration fo symptoms. |
|
|
Salmonella Typhi
|
(AKA S. Typhosa) causes Typhoid fever. The exception, this bug only inhabits humans (not a Zoonosis). S. paratyphi and schotmulleri cause milder types of enteric fevers. Transmission is via direct contact or by infected food/water. Typhoid Mary was a typical carrier (after infection the organism took up residence in the gall bladder and was shed forever.
Virulence- Ingested organisms multiply in the SMALL intestine. They penetrate M cells at the distal ileum and move via lymphatics into the blood (3-4 days of bacteremia). Liver and spleen macrophaged clear the organisms but they are FACULTATIVE INTRACELLULAR PARASITES so they myltiply intracellularly. This allows infection of the gall bladder (carrier) where they gain re-entry into the gut. Carriers can shed organisms for more than a year. The process used by macrophages is "spations phagoytosis" in which "large ruffles" of pseudopodia engulf the bug. Diasease- Enteric fever initially including malaise, headache, and anorexia. 7-10 days: a stepwise rise in fever to 102-104, constipation/diarrhea, BLOOD in stool 2nd week: rose spots on abd, constant high fever, + stool culture, septic @ 14 days. 3rd Week: Fever remits. Organisms shed back to bowel, with ulceration/perforation and resultant BLOODY diarrhea Dx- Culture and serotyping are used. Blood in the 1st week, stool from 2nd week on. The Widal test (rise in S. Typhi agglutinins) is used to confirm a Dx in acute vs. convalescent states (titer not useful in acute phase alone). Treat with short course fluroquinolones. Some multiply resistant strains exist now. Anti Vi and O antibodies are protective, and infection stimulates cellular immunity. Vaccines include Ty21a (live attenuated oral), and Vi (purified capsular polysaccharide) (the killed whole cell had considerable toxicity and is no longer available). |
|
|
Salmonella Choleraesuis
|
Causes septicemic syndrome/bacteremia which is metastatic. Includes spiking fevers, seeding to various organs with resulting abscesses, meningitis, pneumonia, osteomyelitis, and endocarditis. Intestinal symptoms may be absent and stool cultures negative.
|
|
|
ETEC
|
Enterotoxigenic E. Coli casues Trevelers Diarrhea, an ddiarrhea in infants in developing countries. symptoms include watery diarrhea, nausea. abd. cramps, and low grade fever. Rare in developed countries, ETEC spreads via contaminated food/water (no aminal reservoir). They colonize the SMALL intestine using fimbriae (pilli) with colonozing factor antigens (CFA). 2 kinds of toxins are present, Heat labile (LT)which is imunogenically cross reactive with cholera toxin and works in a similar fashion, and heat stabile (ST) which is short and non-immunogenic. Antibodie to CFA and LT are protective, but there is no vaccine.
|
|
|
EPEC
|
Enteropathogenic E. Coli. causes infantile diarrhea in the US and elsewhere. The watery diarrhea is the result of the formation of "attaching and effacing" lesions in which a pedestal forms onthe host cell surface, via actin rearrangement, in contact with the EPEC Bundle-Forming pilli. The protein INtimin then mediates entry into the cell and the microvilli are disrupted (effaced). The mode of transmission is uncertain, but EPEC is identified by Serotyping of O antigen (55, 86, 111, 119, 125, 126, 127, 128, and 142)
|
|
|
EHEC
|
Enterohemorrhagic E. Coli. Causes onset of watery diarrhea and abdominal pain after 4 days of incubation. Bloody diarrhea follows with pus (but not asmuch as shigella). In 10 % of cases in kids hemalytic uremic syndrome results (thrombocytopenia, renal failure, microangiopathic hemolytic anemia). Transmission is via RAW MILK, underooked ground beef, contaminated food (cattle feces) including apples/oranges for drinks and alfalfa sprouts. Cattle are the reservior, and infection is common from petting zoos and person-person. Pathogenesis is the result of attaching and effacing lesions in the large intestine with secretion of Shiga-Like toxin (binds 28S r-rna of 60S unit). The toxin is phage encoded. EHEC O:157 does not ferment sorbitol, so sorbitol containing medium is used for Dx.
|
|
|
EIEC
|
Enteroinvasive E. Coli. Shiga-like invaders of epithelial cells in the LARGE intestine. Cause dysentery with fever, severe abd. cramps, and stool with BLOOD, PUS, and MUCUS. Mostly in developing countries in kids under 5. No animal reservior.
|
|
|
EAEC
|
Enteroagressive E. Coli. Causes prolonged watery diarrhea in some developing countries. Have bundle forming pili like EPEC that cause aggregation. Produce Heat Stabile toxin called EAST whcih elevates cGMP
|
|
|
EHEC
|
Enterohemorrhagic E. Coli. Causes onset of watery diarrhea and abdominal pain after 4 days of incubation. Bloody diarrhea follows with pus (but not asmuch as shigella). In 10 % of cases in kids hemalytic uremic syndrome results (thrombocytopenia, renal failure, microangiopathic hemolytic anemia). Transmission is via RAW MILK, underooked ground beef, contaminated food (cattle feces) including apples/oranges for drinks and alfalfa sprouts. Cattle are the reservior, and infection is common from petting zoos and person-person. Pathogenesis is the result of attaching and effacing lesions in the large intestine with secretion of Shiga-Like toxin (binds 28S r-rna of 60S unit). The toxin is phage encoded. EHEC O:157 does not ferment sorbitol, so sorbitol containing medium is used for Dx.
|
|
|
EIEC
|
Enteroinvasive E. Coli. Shiga-like invaders of epithelial cells in the LARGE intestine. Cause dysentery with fever, severe abd. cramps, and stool with BLOOD, PUS, and MUCUS. Mostly in developing countries in kids under 5. No animal reservior.
|
|
|
EAEC
|
Enteroagressive E. Coli. Causes prolonged watery diarrhea in some developing countries. Have bundle forming pili like EPEC that cause aggregation. Produce Heat Stabile toxin called EAST whcih elevates cGMP
|
|
|
EHEC
|
Enterohemorrhagic E. Coli. Causes onset of watery diarrhea and abdominal pain after 4 days of incubation. Bloody diarrhea follows with pus (but not asmuch as shigella). In 10 % of cases in kids hemalytic uremic syndrome results (thrombocytopenia, renal failure, microangiopathic hemolytic anemia). Transmission is via RAW MILK, underooked ground beef, contaminated food (cattle feces) including apples/oranges for drinks and alfalfa sprouts. Cattle are the reservior, and infection is common from petting zoos and person-person. Pathogenesis is the result of attaching and effacing lesions in the large intestine with secretion of Shiga-Like toxin (binds 28S r-rna of 60S unit). The toxin is phage encoded. EHEC O:157 does not ferment sorbitol, so sorbitol containing medium is used for Dx.
|
|
|
EIEC
|
Enteroinvasive E. Coli. Shiga-like invaders of epithelial cells in the LARGE intestine. Cause dysentery with fever, severe abd. cramps, and stool with BLOOD, PUS, and MUCUS. Mostly in developing countries in kids under 5. No animal reservior.
|
|
|
EAEC
|
Enteroagressive E. Coli. Causes prolonged watery diarrhea in some developing countries. Have bundle forming pili like EPEC that cause aggregation. Produce Heat Stabile toxin called EAST whcih elevates cGMP
|
|
|
Comparison of pathogenic E. Coli
|
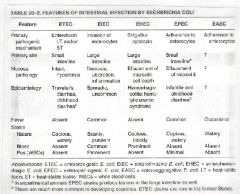
Comparison of pathogenic E. Coli
|
|
|
Yersina Enterocolitica
|
motile gram - rod produces enterocolitis including fever, diarrhea, and abdomimal pain. Nonenteric lymphadenitis and septicemia are common. 2 modes of pathogenesis- incasion of the M cells to facilitate escape from the gut (similar to S. Typhi) and secretion of toxin similar to E. Coli ST which casues diarrhea. Farm animals are the reservoir.
|
|
|
Summary of Enteric Toxins
|
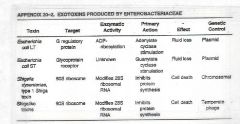
Summary of Enteric Toxins
|
|
|
Endogenous Vs. Exogenous anaerobic infections
|
Endogenous- 95% of anaerobic infections are by non-spore forming anaerobes in mixtures (polymicrobic) containing normal flora (anaerobes and facultatives/aerobes)
Exogenous anaerobic infections account fro 5% total, by clostridium (spore formers) following ingestion or traumatic exposure to contaminants. Note that Stools are mostly gram +, Pus from abscess is mostly gram -, so that there are different bugs present. |
|
|
Anaerobic Bacteria (Definition)
|
Gram +/- in various morphologies. Require 2-7 days to incubate, anaerobically or course. THEY WILL NOT GROW IN 10% O2. Life without O2 (anaerobiosis) causes them to use fermentative reactions for energy, and non O2 electron donors/acceptors for energy production. They WILL NOT grow in a candle jar as in spite of its 3-5% CO2 there is still 15-17% O2. There are grades of oxygen sensitivity (strict anaerobes, microaerophilics, and aerotolerants) Some are also capnophilc (require increased CO2 to grow. For anaerobes, O2 is lethal as they cannot detoxify speroxide, H2O2, or hydroxyl radicals as they lack superoxide dismutase and catalse (bacteroids fragilis being the exception). The theory behind O2 toxicityis that electrons are shunted from energy production to reduce O2, causing growth to stop (recoverable static phase), however continued exposure produces a cidal (irreversable) phase as excess radicals build.
|
|
|
Growth of Anarobes
|
Grow in anaerobe jar or anaerobic glove box on agar surface or in prereduced liquid medium. A gas pak in a sealed jar or mix from a gas tank producing a reduced athmosphere of 85% N2, 10% H2, and 5% CO2. Results in a LOW Eh (O/R potential = -175 to -250 mV, permitting growth similar to necrotic/gangrenous tissue, = +100 in Living tissue). They requre rich media including blood/hemin, a fermentable carbon source, Growth factors (vitamin K), and Reducing agents to maintain Low Eh. Proper specimen collection and culturing to minimize O2 is important, rapid transport to the Lab. Purulent material may kill anaerobes as it contains H2O2. Results in 3-7 days, expensive tests, so use clues to be sure that anaerobes are present.
|
|
|
Locaton of anaerobes
|
In Nature: ubiquitous in soil, fresh and salt water, mud, etc. These casue exogenous infections (although there are some bacteria that can cause both exogenous and endogenous infections including Clostridium [spores]).
In Humans/animals: 4 major anatomical sites and in feces (over 99% of normal fecal flora are anaerobes. This is the source of endogenous auto-infections. The normal locatoin of anaerobes is indicitive of where they cause mixed infections when they transgress epithelial or mucosal barriers. There are hundreds of species, and they grow synergistically wtih facultatives/normal flora like strep and staph. 4 sites: Oropharynx/Upper respiratory tract- They outnumber facultatives 10:1 (oral cavity) to 1000:1. GI Tract- 1000X as many anaerobes as facultatives. Primarily in the colon, few above the terminal ileum. 99% are Gram - (mostly bacteroides and less fusobacterium) with soem gram positives (like peptosterptococcus). 1% clostridium species. They all constitute normal fecal flora. Female Genital Tract- Teh vaginal mucosa shows about a 1:1 ratio Skin- About a 10:1 ratio. Propionibacterium acnes is found deep in sebaceous gland ducts/hair follicles (associated with acne, NOT CHOCOLATE) as there are layers of dead cells with nooks and crannies, areas of low Eh. Note that most of these are gram -'ves. |
|
|
Anaerobes of the Oropharynx/upper respiratory tract
|
Pigmented PREVOTELLA and PORYPHYROMONAS (G- rods)
Bacteroides oralis group (Nonpigmented G- rods) FUSOBACTERIUM nucleatum (G- rods) PEPTOSTREPTOCOCCUS (G+ cocci) ACTINOMYCES (G+ rods) Veillonella (G- diplococci) |
|
|
Anaerobes of the Female Urogeital tract
|
PIGMENTED PREVOTELLA and PORPHYROMONAS (G- rods)
PEPTOSTREPTOCOCCUS (G- rods) lactobacillus (g+ roda) |
|
|
Anaerobes of the Colon
|
ACTINOMYCES (G+ rods)
BACTEROIDES FRAGILIS GROUP (nonpigmented G- rods, worst of all endogenous causes. Member of the Bacteroidaceae family, most common among Normal GI flora. Normally provides us with our Vit K) clostridium (G+ rods) FUSOBACTERIUM (G- rods) PEPTOSTREPTOOCCUS eubacterium (G+ rods) |
|
|
Anaerobes of the Skin
|
propionibacterium acnes (G+ rods, chocolate is not for acne or sex... milk hormones predisposes of sebum production. They grow with yeast and clog the gland to WBC's come and form pus. Spicy foods containing iodides, shellfish, soy sauce also contribute)
|
|
|
Features of mixed anaerobic infections
|
Bacteroides (+ prevotella and porphyromonas), Fusobacterium, and peptostreptococcus are the most common causes for endogenously aquired abscesses or empyemas (pus in a cavity). Facultatives may or may not be involved.
Anaerobes are the maor agents in chronic sinusitis, otits media, aspiration and necrotizing pneumonia, bronchiactysis, cholecystitis, septic arthtritis, osteomyelitis, and decubitus ulcers. They are rare in UTI's. They are also involved in brain abscesses, upper and lower resp tract infections, intraabdominal/OB/GYN/Pelvic infections and soft tissue infections. Bacteremia, endocarditis, and oral infections as well. In mixed infections, esp those originating below the diaphragm (enteric) normal faculatatives get inolved (including coliforms and enterococcus faecalis). abscesses are common. These situations require a 2-3 antibiotic regimen. Pure (single species) infections are usually associated with normally sterile, closed spaces or organs such as joint cavities, brain, CNS, blood stream. |
|
|
Porphyromonas Gingivalis
|
causes periodontal disease and is a risk factor for preterm labor/low birth weight babies, CV disease, stroke, diabetes. It is a component of plaque biofilms and has fimbrae and a polysaccharide capsule to aid in adherence. It invades epithelial and endothelial cells where it replicates, produces proteases (degrade Ig and complement) and induces markers of inflamation associated with coronary artery disease.
|
|
|
Exogenous origen of anaerobic infections.
|
Clostridium is of significance as the endospores that it forms are heat, dessicatin, UV, and Chamical resistant and may persist for years (autoclaving is effective to remove them through). Thus they can cause exogenous or endogenous infections.
|
|
|
Important Anaerobes to know
|
These (except for actinomyces) account for 1/2 - 2/3 of all isolates form anaerobic infections.
Bacteroides Fragilis Group- 10 species, G- rods, non pigmentd, localized abscesses etc... Prevotella and Prophyromonas (originally though to be bacteroides too) are in the ororespiratory/dental infections as well as elsewhere. G- rods Fusobacterium- G- rods of the bacteroides species Clostridium species- G+ rods, the only spore forming anaerobe peptostreptococcus species- G+ strep, abscess formation etc... actinomyces israelii- G+ rods, infections are less common |
|
|
Predisposing factors in anaerobic infections
|
1. Trauma or ischemia- decreased blood/Os supply lowers O/R potential. Ie...Rupture/perf releases aerotolerant and anaerobes,the former protecting the latter, leading to abscess formation and further tissue necrosis.
2. Diabetes/debilitations compromising circulation. Also true of alcoholics and pt's with diseases like diverticulitis 3. Immunologic defects (congenital, leukemia, chemo-induced) 4. Physician induced factors- inappropriate use of antibiotics can lead to anaerobic infections. Also surgery can seed peritoneal cavities, muscles, or blood with anaerobes. General anesthesia can lead to pneumonias/lung abscesses due to aspiration of oral anaerobes (also via aspiration of vomitus). 5. Pressure necrosis/stenosis of "tubes" of the body. Tumorsof the larynx/pharynx can occlude the airway, so bacteria are not removed efficiently by mucus and settle deep in lung. 6. Anaerobic abscesses can extend causing pressure necrosis or seeding. Gram - rods are recovered in 10% of all bacteremias. Reduced perfusion, necrosis, and low Eh can also interfere with antibiotic delivery. Note that the oxygen dependant leukocyte bacteriocidal acrivity fails in the anaerobic conditions of an abscess. 5. |
|
|
Clinical Clues of anaerobic sepsis
|
Tx can't wait for confirmation via lab culture, so the clues are:
1. Infections associated with the 4 body sites normally harborig anaerobes. (often continuus with mucoas, form in closed spaces as abscesses or burrow through tissue. 2. Foul smelling (fetid) odor(cadaverine/putracine) with discharge/pus. 3. Tissue necrosis with gangrene 4. Infection with gas production(fermentation) causes crepitus tissue (crackling on palpation) 5. Gram stain of exudate shows characteristic mixed flora, heavy on the -'ves, pleiomorphic rod/filamentous or coccobacillay shapes. 6. failure to recover pathogens form aerobic culture when signs of infection present. 7. Failure to respond to antibiotics not active against anaerobes. Resistant strains of anaerobes are appearings but many are still susceptable to Pcn G, esp in the oral cavity. 8. Thrombophlebitis of unknown etiology. Could be that that body's heparin has been degraded by heparinase found in some anaerobes, or endotoxin of gram -'ves activating the clotting cascade. 9. Oral infections- root canal/endodontic lesions and gum (periodontal) disease are associated with mixed anaerobes. |
|
|
Determinants of anaerobic pathogenicity
|
1. Clostridial species: each has it own exotoxins and uses small organic molecules as e- aceptors rather than O2. Spores are hardy but inert until they germinate. The other medically important spore former is Bacillus Anthracis. Also a G+ rod, but aerobic.
2. Mixed infections of endogenous origens (where clostridia are not involved. One anaerobe can't be characterized as the etiologic agent, but those with greatest virulence seem to multiply when the Eh is lowered appropriately. The O2 sensitive and avirulent flora are cleared by host defences in the case of sterile tissue invasion, leavin the pathogenic ones to destroy. Likely culprits here are Bacteroides, fusobacterium, peptostreptococcus, porphyromonas, prevotella, and propionibacterium. They can act together synergisticaly thus: a. Gram - anaerobes contain endotoxin 9although Bacteroides Fragilis contain lipid A which LACKS 2 keto 3 deoxyoctolusonic acid (KDO) and is thus less potent b. Prevotella produces a collagenase and its capsule promotes adherence to peritoneum (pulmonary abscess above diaphragm) While prevotella and porphyromonas produce proteases against IgA, IgM, and IgG. c. Fusobacterium produces hemolysin, lysases, and lectins d. Some anaerobes produce PL-C, proteases, neuraminidase, hemolysis, collagenases, fibrinolysin, heparinase, chondroitin, sulfatase, glucuronidases, NAGinidase, and volatile fatty acids. e. Bacteroide Fragilis resides at mucosal sites adjascent to the infections and gain access. Then they produce adhesins, resist O2 toxicity, produce MANY virulence factors, and are antiphagocytis and tissue necrotic. 3. host immunity: humeral and cellular immunity are bothinvolved in a patients response to an anaerobe mediated infection. They are not very effective against abscesses through. Many anaerobes have serum independant chemotactic factos that attract PMN's, so they are opsonized by the alternative complement pathway and phagocytosed. Also, B fragilis is susceptable to the classic pathway, but does not confer lasting immunity. Note that passive antibody transfer will protect from bacteremia but T-Dependant responces may be necessary for protection from abscess formation. |
|
|
Bacteroides Fragilis
|
Group members normally reside at mucosal sites adjascent to infections. When they gain access they make all kinds of stuff including:
Heparinase - resulting in thrombophlebitis Superoxide dismutase and catalse- that cna survive for days in air and are protective Polysaccharide capsule (some strains) of agglutinin tha tis antiphagocytotic and anti-chemotactic. Also aids in attachment and abascess formation. A MAJOR virulence factor with the surface pilli Histolytic enzymes (hyaluronidase, collagensae, chondroitin sulfatase, fibrinolysin, neuraminidaese, DNAse Lipipolysaccharide- lacks endotoic properties as it has less lipid, however it stimulates leukoyte migration and chemotaxic by the alternate pathway. Also antiphagocytic. Some species do have entotoxic LPS that can lead to shock. It can transfer genes for drug resistance via plasmids and transposons to other bugs. All strains produce B-lactimase (inactivates most penicillins...unique to this group). also resistant to many cephalosporins and tetracyclines. Clindamycin resistance (plasmid mediated) is on the rise. some species produce IgA protease, Heparinase, DNase, and fimbriae which aid in adherence. It makes vitamin K (not a virulence factor) note that asit resides below the diaphram most of the abscesses are abdominal. 1/2 - 2/3 of all clinically significant anaerobic isolates are members of this group, and the pigmented anaerobic gram- bacilli (other bacteroides + porphyromonas). More than 80% of the intraabdominal infections are associated with B fragilis (even though it is only a minor member of the GI tract flora. Deep pain and tenderness below the diaphragm is typical of infection, often with fever in acute intra abdominal abscess. These characteristics help determine treatment. B. Fragilis members spread to the bloodstream more than any other anearobe. They also have the most antibotic resistance (generally they are susceptable to metronidazole, clindamycins, and 3rd generation cephalosporins, but not PCN. This is used diagnostically (resistance to a 1000 mcg kanamycin disk, also vanco and colistin, and are stimulated by 20% bile). other kinds of bacteroides may be involved in aspiration pneumonia, as well as brain abscesses (along with S. Aureus) |
|
|
Tx/Prophylaxis for endogenous anaerobic infections
|
Prophylaxis- clean wounds and debride (remove tissue with low O/R potential).
Tx- surgical drainage of of the lesion combined with antibiotics. Base prsumptive Dx on clinical clues, gram stain, and prediction. Seriously ill pt's get antibiotics against both aerobes/facultative and anaerobes (2-3 drug combo). antimicrobials include Pcn and Cefoxitin, however as some resistance to both is possible, sensitivity testing may be indicated. The first line for intraabdominal/pelvic or other serious anaerobic infetion is metronidazole (flagyl), often used with cefoxitin and an aminoglycoside (for facultatives and G- enterics). (clindamycin may also be used, esp for infectons above the diaphragm, although there is some resistance.) Oral infections can be treated with PCN. |
|
|
Actinomyces Israelii
|
Gram + rod Endogenous infection (often mixed) with normal anaerobic flora of oral cavity/oropharynx/colon. They are non-acid fast, microaerophilic to anaerobic with branching beaded filaments. Note that they reproduce by forming arthrospores (NON-RESISTANT fragments of filament). They are distinguished from Nocardia which appear similar but are partially acid fast aerobes. There is no human transmission, infections are endogenous.
Source of virulence is unclear. They are related to mycobacterium, so hypersensitivity may be important. No toxins. Actinomycosis is characterized as a slow burrowing infection, often a result of interruption of the mucosa producing local inflamation and infection (surgical or traumatic). Initial presentation usually includes an abscess in the mouth, jaw, lungs, Gi or Female Gu tract. It is then characterized by granuloma formation with suppuration and abscess that drain via burrowed sinuses to the outside. The sinuses are filled with PMN's surrounded by fibrous tissue, they do not heal, and discharge pus containg "sulfur granules(diagnostic)." The granules contain no sulfur (they are yellow), but have actinomyces at the center surrounded by an eosinophilis protinaceous coat and inflamatory cells. 3 forms of the disease are: Cervicofacial: Most common, swelling of jaw, face, neck, with bone and soft tissue involvement. rarely may include CNS infection/meningitis assoiated with trauma to the jaw/gingiva/Gi tract. Thoracic: rare, affecting the lungs and thoracic cage. A result of aspiration. Rarely can lead to brain abscesses. Abdominal: Rare (via escape from colon is also rare). affectins bowel/pelvis, and can be associated with IUD's or surgery. Note that it is often mistaken for a neoplasm, and men are 3X more likely to get it, but IUD use is a risk factor. Tx-High doses of PCN-G for 1-18 months, and possible surgical drainage and debridement. |
|
|
Nocardia
|
Resembles actinomyces morphologically (Gra + rods) but are aerobic, and weakly acid fast. They are found in the soil (cause only exogenous infection)
Norcardiosis includes infections of the lung and brain following inhalation of the bacteria, or mycetomas and pustules of the foot following injury. Both are chronic, granulomatous diseases. The pulmonary form is most common in immunocompromised/transplant patients. Tx- they are PCn resistant and as such TMP-Sulfa or another combo is often used. Humoral immunity is not effetive, however T-cell mediated immunity is effective, and when it is impaired pt's are at greater risk |
|
|
Clostridium Tetani
|
Motile Gram + rod, tennis raquet shaped (terminal endospore). Those at risk are neonates (spores enter cut umbilical cord), elderly (questionalbe immunizations), severe burn pt's, and IV drug users. Infection is usually via laceration/puncture wound, severe burn, abortion, and birth (neonate and mom).
Virulence- Spores that are part of a mixed infection germinate when aerobes devitalize the tissue. They form bacilli, grow, enter stationary phase, and lyse releasing a plasmid encoding toxin. The pathogen is non-invasive, but the Tetanospasmin (toxin) is very potent/dangerous. Its B subunit binds to a presynaptic nerve terminal and the A unit is taken via retrograde txport to the cell body (in the CNS) where via proteases it blocks the release of GABA and Glycine (inhibitory neurotransmitters) resulting in inreased action of muscles. (again note that the toxin needs to travel from the periphery to the CNS to act, also the binding is irreersable so we need to generate new nerve terminals to fully releive symptoms.). Diagnosis is generally based on clinical findings as organisms recovery is difficult (they are sensitive to O2 and the wound may be elusive) Tetanus (symptoms): Spastic Paralysis (unregulated synaptic activity provked by minor stimuli) Trismus (inability to open mouth) Risus Sardonicus (Sardonic smile with drooling) Lockjaw Opisthotonus (back spasm arching the head, neck, and spine) Convulsions (exhaustion, respritory muscle paralysis, death) Tx- immediately clean wound and start prophylaxis. Alternate site injection fo Toxoid booster and Human antitoxin (HTIG). Both are necessary as the incubation for wounds near the brain is 3-14 days, but 4-5 weeks for those elsewhere, so we need short term and long term protection. Note that HTIG is human so it does not cause hypersensitivity. Additionally surgical debridement, removal of stimuli( quiet, darkness), muscle relaxants/GABA agonists can help. Incidence is low as a result of the DTaP vaccine and antitoxin. The vaccine is efective, bu toxoid boosters should be given at intervals throughout life as failure to do so compromised immunity (give booster with a clean wound if >10 yrs since last one, >5 years for all other wounds). Antibiotic therapy is of questionable value. |
|
|
Clostridium Perfringens
|
Spore forming (rarely seen in gram stain) box car shaped G+ rods, encapsulated (diagnostic). In smears where it is present, there are no inflamatory cells as a result of the alpha toxin, however on blood there is a double concentric hemolytic zone (alpha and theta toxin), and on egg yolk agar liids in serum and yolk are hydrolized producing a precipitate. There are 5 subtypes, A-E with A the most common, in colon and soil.
It forms a variety (12) of toxins and enzymes after germination: Alpha Toxin- THE MOST IMPORTANT, a lecithinase/PLC which lyses cell membranes (esp muscles, WBC's, RBC's). It is a dimer with A and B units, and causes terrible pain. Theta toxin- alters capillary membrane, hemolytic and necrotizing. Also is cardiotoxic (not a lecithinase) Collaginase, Fibrinolysin, neuraminidase, Dnase, Hyaluronidase (spreading factor), and proteases...all liquify tissue and facilitate spread. Acid and Gas from metabolism Diseases: anaerobic cellulitis- inflamation of CT between adjascent tissues and organs, may spread to blood. Important in diabetic patients. Fasciitis- facial planes involve. Clostridial endometritis- inflamation of membrane lining wound following physical damage Non-fatal food poisoning- Usually type A, but other species cause it as well. High incidence associated with restaurantes etc where large quantities of food are served. Common vectors include beef or poultry gravies or stews not refrigerated after cooking. Ingested spores germinate in intestines and produce heat labile extoxin (enterotoxin) causing gastroenteritis 8-24 hours after ingestion. Symptoms include mild GI distress, diarrhea, cramps, nausea (no fever and rarely vomiting). Similar to cholera and ETEC, ion transport is interrupted and membrane permeability altered/pores are formed. Also epithelium is damaged and glucose uptake is inhibited. Increased cAMP responsible for loss of fluids. The disease passes after 12-24 hours requiring only fluid and electrolyte replacement (esp in young and elderly). May be responsbile for some Antibiotic Induced Diarrhea as it is normally present in low numbers. Gas Gangrene (AKA clostridial myonecrosis) is common in untreated war wounds (soil/fecal contamination), deep muscel lasceration, crushing injury, post partum contamination, post operative (especially gall Bladder), or Txplant tissue contamination (knee ligament). 40-100% fatal even with treatment. Can develop in a few hours with Dx via clinical presentation (although organisms will be isolated 80% of the time). Progresses quickly- INTENSE pain, muscle necrosis, spreading hemolysis, foul/fetid odor, green/grey coloration, and crepitance. A black exudate and black urine may also result. Leads to shock, renal failture, tachycardia, hemolytic anemia, and death in about 2 days or less. Tx- gas gangrene immediately with debridement/amputation, hyperbaric oxygen (speeds healing, inactivates theta toxin, does not kill organism though) There is no vaccine or useful antitoxin. |
|
|
Clostridium Difficile
|
Toxigenic and non-toxigenic strains are endogenous in small numbers in 2-3% of healthy adults. It can be passed between patients of via spores in the environment.
Virulence: overgrowth occurs via elimination of competing flora (uaually outnumbered 1000:1) which can be caused by antibiotics (esp clindamycin, ampicillin, and cephalosporins as well as some antineoplastics), and liberal use of proton pump inhibitors. This is the most common cause of diarrhea in hospital patients and those on Antimicrobials. C. Diff produces 2 toxins... Exotoxin A is similar to an enterotoxin, altering membrane permeability (fluid secretion) and causing epithelial/mucosal cell necrosis, abd pain, inflamation and fever. Exotoxin B is a cytotoxin killing epithelial cells. It is 100 X more potent and A, but may use A in that the mucosal damage A causes allows for uptake of B. Diseases: Overgrowth of toxigenic strains produces Antibiotis associated diarrhea (AAD) or Pseudomembranous colitis (PMC). [note that they can also becaused by C. Perfringens etc]. AAD- milder disease wtih varying inflamation. C-Diff causes about 25%. Onset after 5-10 days of drug tx or less, potentially life threatening in the sick and elderly. Pseudomembranous Colitis- Causes abrupt bloody diarrhea with abd pain, fever. The pseudomembrane exudate has mucus, fibrin, inflamatory cells, RBC's and cell debris, and is best visualized via colonoscopy or endoscopy (yellow/white plaques). Can lead to colon Perf and fatal sepsis. Can be the post-op effect of pre-op prophylaxis. Tx. Dx is generally vis detection of TOXIN [B via culture and cytotoxic assay, A and B via ELISA](not cells) in the stool as it is normal flora so we expect the bug itself to be there. Highly lethal strains (B1) are resistant to fluroquinolones. Both A and B toxins produce and antibody response, which immunity may help resolve the disease. Also stop the offending antibiotic, replace fluis where indicated and give vancomyocin and metronidazole(preferred) for 2 week. 20% of patients relapse. eating yogurt can help, but avoid antidiarrheal meds which actually prolong the disease. |
|
|
Clostridium Botulinum
|
causes 3 major kinds of botulism:
Food Poisoning (rare today, usually fatal), wound botulism (rare), and infant botulism (now the most common. Serotypes A, B, E, and occationally F cause human outbreaks, along with C. baratti and C. Butyricum which also produce the toxin. Food Botulism: Preformed toxin is ingested with contaminated food and absorbed. Many can get sick from a single food source, usually cans or blister packs of fish with anaerobic conditions. Spores are presentin soil and oceans comtaminating food and fish. Note that the toxin is destroyed by 10 minutes of boiling but the spores resist for up to 5 hours. The toxin itself is a dimeric A-B type that acts as a neurotoxin. 7 subtypes exist (A-G with A, B, and E the most common in humans, C in fowl, D in callte). In some strains production is controlled by lysogenic conversion, in others by a plasmid or chromosomal gene. The toxin is resistant to digestive tract acids causing intoxicaiton without infection, and <1ug kills!!! 18-92 hours after ingestion it acts on the PNS by binding to cholinergic nerves and blocking the release of ACh (prevents exocytosis). This results in flaccid muscle paralysis (both voluntary and autonomic function is effected) leading to cardiac arrythmias and BP changes. Other symptoms include diplopia, dysphageia, constipation, and abdominal pain (no fever or sepsis). Death is the result of respiratory muscle paralysis. Tx via injection of specific type or trivalent horse antitoxin (to all who ate the meal) and consider mechanical ventilation to peclude respiratory failure. The specific toxin can be isolated form serum orthe source food. Adequare pressure cooking/autoclaind during canning kills spores. If you MUST eat suspect food boil it for 10 minutes. Refrigerate foods after cooking. There is no vaccine. Infant Botulism (AKA Floopy Baby Syndrome)- Mostly type A or B CB in 2-4 month olds. spores from some formulae, honey, dust, or soil are ingested and germinate, coloniing the colon where exotoxin gets absorbed (Infections and Intoxication). Presents with several days of constipation followed gy flaccid paralysis/floopy state, ptosis, dialated pupils, lethargy, disphagia, weak cry, respiratory difficulties (can be fatal) [in all a descending paralysis]. Presence of toxin in food or feces aids in Dx [cytopathic effect on culture cells or ELISA, or mouse injection, orgnaism culture is rare]. Tx via prolonges mechanical ventilation and symptomatic. No horse antitoxin to prevent anaphylaxis or lifelong hypersentisivity. Human is available for Infant botulism only! Antibiotics are not useful. Generally they recover but not always, one possible casue of SIDS. Wound Botulism- rare, usually in IV drug users or contaminated wounds. Pcnis useful to tx as is horse Ab (the only variety where antibiotics is prescribed). Symptoms are as described above. Botulinum toxin A (botox) or B (myoblock) have medical uses as well. Muscel spasms, strabismus, facial pain from trigemnal neuralgia etc. Also used cosmetically. Aerosolized spres may be among hte greatest bioterrism threats, whereas the most common is toxin added to food and drink. Beware and report b/l symmetrical descending paraysis with respiratory failure 1-5 days after eating the contaminant. CDC gives trivalent horse Antitoxin, and the DOD has heptavalent. Check for hypersensitivity first. |
|
|
Anaerobic Specimen Collection, transport, and ID
|
Collection-
blood culturs taken into anerobic media (blood bottles), aspirates of closed space infections, use syringe for collection (invasive procedure. Swabs are worst as they will pick up much normal flora. Inappropriate specimens to give meaningless into expectorated sputum ,throat swabs, nasal discharge, feces, vaginal swabs, voided urine, ileostomy/colostomy effluent an dbronchoscopy aspirates. Transport- rapid is important to rpevent overgrowth by facultatives. prefer liquid or tissue specimens, preferably bloody. Swabs as a last resort. Transport media for this purpose to retain unaltered proportans of viable organisms. Identification- based on morphology, gram stain, biochemical tests, and biochemical tests. can use gas-liquid chromatography etc...but gram staining is likely the most useful info for a quck clinicl decision. Card type tests are fast andsophisticated by expensive. Antibiotic sensitivity tests are not done as patterns are already established, so that we need to examine clues. We use the fast E-test for sensitivity of anaerobes if anything. |
|
|
Actinomyces Israelii
|
Gram + rod Endogenous infection (often mixed) with normal anaerobic flora of oral cavity/oropharynx/colon. They are non-acid fast, microaerophilic to anaerobic with branching beaded filaments. Note that they reproduce by forming arthrospores (NON-RESISTANT fragments of filament). They are distinguished from Nocardia which appear similar but are partially acid fast aerobes. There is no human transmission, infections are endogenous.
Source of virulence is unclear. They are related to mycobacterium, so hypersensitivity may be important. No toxins. Actinomycosis is characterized as a slow burrowing infection, often a result of interruption of the mucosa producing local inflamation and infection (surgical or traumatic). Initial presentation usually includes an abscess in the mouth, jaw, lungs, Gi or Female Gu tract. It is then characterized by granuloma formation with suppuration and abscess that drain via burrowed sinuses to the outside. The sinuses are filled with PMN's surrounded by fibrous tissue, they do not heal, and discharge pus containg "sulfur granules(diagnostic)." The granules contain no sulfur (they are yellow), but have actinomyces at the center surrounded by an eosinophilis protinaceous coat and inflamatory cells. 3 forms of the disease are: Cervicofacial: Most common, swelling of jaw, face, neck, with bone and soft tissue involvement. rarely may include CNS infection/meningitis assoiated with trauma to the jaw/gingiva/Gi tract. Thoracic: rare, affecting the lungs and thoracic cage. A result of aspiration. Rarely can lead to brain abscesses. Abdominal: Rare (via escape from colon is also rare). affectins bowel/pelvis, and can be associated with IUD's or surgery. Note that it is often mistaken for a neoplasm, and men are 3X more likely to get it, but IUD use is a risk factor. Tx-High doses of PCN-G for 1-18 months, and possible surgical drainage and debridement. |
|
|
Nocardia
|
Resembles actinomyces morphologically (Gra + rods) but are aerobic, and weakly acid fast. They are found in the soil (cause only exogenous infection)
Norcardiosis includes infections of the lung and brain following inhalation of the bacteria, or mycetomas and pustules of the foot following injury. Both are chronic, granulomatous diseases. The pulmonary form is most common in immunocompromised/transplant patients. Tx- they are PCn resistant and as such TMP-Sulfa or another combo is often used. Humoral immunity is not effetive, however T-cell mediated immunity is effective, and when it is impaired pt's are at greater risk |
|
|
Clostridium Tetani
|
Motile Gram + rod, tennis raquet shaped (terminal endospore). Those at risk are neonates (spores enter cut umbilical cord), elderly (questionalbe immunizations), severe burn pt's, and IV drug users. Infection is usually via laceration/puncture wound, severe burn, abortion, and birth (neonate and mom).
Virulence- Spores that are part of a mixed infection germinate when aerobes devitalize the tissue. They form bacilli, grow, enter stationary phase, and lyse releasing a plasmid encoding toxin. The pathogen is non-invasive, but the Tetanospasmin (toxin) is very potent/dangerous. Its B subunit binds to a presynaptic nerve terminal and the A unit is taken via retrograde txport to the cell body (in the CNS) where via proteases it blocks the release of GABA and Glycine (inhibitory neurotransmitters) resulting in inreased action of muscles. (again note that the toxin needs to travel from the periphery to the CNS to act, also the binding is irreersable so we need to generate new nerve terminals to fully releive symptoms.). Diagnosis is generally based on clinical findings as organisms recovery is difficult (they are sensitive to O2 and the wound may be elusive) Tetanus (symptoms): Spastic Paralysis (unregulated synaptic activity provked by minor stimuli) Trismus (inability to open mouth) Risus Sardonicus (Sardonic smile with drooling) Lockjaw Opisthotonus (back spasm arching the head, neck, and spine) Convulsions (exhaustion, respritory muscle paralysis, death) Tx- immediately clean wound and start prophylaxis. Alternate site injection fo Toxoid booster and Human antitoxin (HTIG). Both are necessary as the incubation for wounds near the brain is 3-14 days, but 4-5 weeks for those elsewhere, so we need short term and long term protection. Note that HTIG is human so it does not cause hypersensitivity. Additionally surgical debridement, removal of stimuli( quiet, darkness), muscle relaxants/GABA agonists can help. Incidence is low as a result of the DTaP vaccine and antitoxin. The vaccine is efective, bu toxoid boosters should be given at intervals throughout life as failure to do so compromised immunity (give booster with a clean wound if >10 yrs since last one, >5 years for all other wounds). Antibiotic therapy is of questionable value. |
|
|
Clostridium Perfringens
|
Spore forming (rarely seen in gram stain) box car shaped G+ rods, encapsulated (diagnostic). In smears where it is present, there are no inflamatory cells as a result of the alpha toxin, however on blood there is a double concentric hemolytic zone (alpha and theta toxin), and on egg yolk agar liids in serum and yolk are hydrolized producing a precipitate. There are 5 subtypes, A-E with A the most common, in colon and soil.
It forms a variety (12) of toxins and enzymes after germination: Alpha Toxin- THE MOST IMPORTANT, a lecithinase/PLC which lyses cell membranes (esp muscles, WBC's, RBC's). It is a dimer with A and B units, and causes terrible pain. Theta toxin- alters capillary membrane, hemolytic and necrotizing. Also is cardiotoxic (not a lecithinase) Collaginase, Fibrinolysin, neuraminidase, Dnase, Hyaluronidase (spreading factor), and proteases...all liquify tissue and facilitate spread. Acid and Gas from metabolism Diseases: anaerobic cellulitis- inflamation of CT between adjascent tissues and organs, may spread to blood. Important in diabetic patients. Fasciitis- facial planes involve. Clostridial endometritis- inflamation of membrane lining wound following physical damage Non-fatal food poisoning- Usually type A, but other species cause it as well. High incidence associated with restaurantes etc where large quantities of food are served. Common vectors include beef or poultry gravies or stews not refrigerated after cooking. Ingested spores germinate in intestines and produce heat labile extoxin (enterotoxin) causing gastroenteritis 8-24 hours after ingestion. Symptoms include mild GI distress, diarrhea, cramps, nausea (no fever and rarely vomiting). Similar to cholera and ETEC, ion transport is interrupted and membrane permeability altered/pores are formed. Also epithelium is damaged and glucose uptake is inhibited. Increased cAMP responsible for loss of fluids. The disease passes after 12-24 hours requiring only fluid and electrolyte replacement (esp in young and elderly). May be responsbile for some Antibiotic Induced Diarrhea as it is normally present in low numbers. Gas Gangrene (AKA clostridial myonecrosis) is common in untreated war wounds (soil/fecal contamination), deep muscel lasceration, crushing injury, post partum contamination, post operative (especially gall Bladder), or Txplant tissue contamination (knee ligament). 40-100% fatal even with treatment. Can develop in a few hours with Dx via clinical presentation (although organisms will be isolated 80% of the time). Progresses quickly- INTENSE pain, muscle necrosis, spreading hemolysis, foul/fetid odor, green/grey coloration, and crepitance. A black exudate and black urine may also result. Leads to shock, renal failture, tachycardia, hemolytic anemia, and death in about 2 days or less. Tx- gas gangrene immediately with debridement/amputation, hyperbaric oxygen (speeds healing, inactivates theta toxin, does not kill organism though) There is no vaccine or useful antitoxin. |
|
|
Clostridium Difficile
|
Toxigenic and non-toxigenic strains are endogenous in small numbers in 2-3% of healthy adults. It can be passed between patients of via spores in the environment.
Virulence: overgrowth occurs via elimination of competing flora (uaually outnumbered 1000:1) which can be caused by antibiotics (esp clindamycin, ampicillin, and cephalosporins as well as some antineoplastics), and liberal use of proton pump inhibitors. This is the most common cause of diarrhea in hospital patients and those on Antimicrobials. C. Diff produces 2 toxins... Exotoxin A is similar to an enterotoxin, altering membrane permeability (fluid secretion) and causing epithelial/mucosal cell necrosis, abd pain, inflamation and fever. Exotoxin B is a cytotoxin killing epithelial cells. It is 100 X more potent and A, but may use A in that the mucosal damage A causes allows for uptake of B. Diseases: Overgrowth of toxigenic strains produces Antibiotis associated diarrhea (AAD) or Pseudomembranous colitis (PMC). [note that they can also becaused by C. Perfringens etc]. AAD- milder disease wtih varying inflamation. C-Diff causes about 25%. Onset after 5-10 days of drug tx or less, potentially life threatening in the sick and elderly. Pseudomembranous Colitis- Causes abrupt bloody diarrhea with abd pain, fever. The pseudomembrane exudate has mucus, fibrin, inflamatory cells, RBC's and cell debris, and is best visualized via colonoscopy or endoscopy (yellow/white plaques). Can lead to colon Perf and fatal sepsis. Can be the post-op effect of pre-op prophylaxis. Tx. Dx is generally vis detection of TOXIN [B via culture and cytotoxic assay, A and B via ELISA](not cells) in the stool as it is normal flora so we expect the bug itself to be there. Highly lethal strains (B1) are resistant to fluroquinolones. Both A and B toxins produce and antibody response, which immunity may help resolve the disease. Also stop the offending antibiotic, replace fluis where indicated and give vancomyocin and metronidazole(preferred) for 2 week. 20% of patients relapse. eating yogurt can help, but avoid antidiarrheal meds which actually prolong the disease. |
|
|
Clostridium Botulinum
|
causes 3 major kinds of botulism:
Food Poisoning (rare today, usually fatal), wound botulism (rare), and infant botulism (now the most common. Serotypes A, B, E, and occationally F cause human outbreaks, along with C. baratti and C. Butyricum which also produce the toxin. Food Botulism: Preformed toxin is ingested with contaminated food and absorbed. Many can get sick from a single food source, usually cans or blister packs of fish with anaerobic conditions. Spores are presentin soil and oceans comtaminating food and fish. Note that the toxin is destroyed by 10 minutes of boiling but the spores resist for up to 5 hours. The toxin itself is a dimeric A-B type that acts as a neurotoxin. 7 subtypes exist (A-G with A, B, and E the most common in humans, C in fowl, D in callte). In some strains production is controlled by lysogenic conversion, in others by a plasmid or chromosomal gene. The toxin is resistant to digestive tract acids causing intoxicaiton without infection, and <1ug kills!!! 18-92 hours after ingestion it acts on the PNS by binding to cholinergic nerves and blocking the release of ACh (prevents exocytosis). This results in flaccid muscle paralysis (both voluntary and autonomic function is effected) leading to cardiac arrythmias and BP changes. Other symptoms include diplopia, dysphageia, constipation, and abdominal pain (no fever or sepsis). Death is the result of respiratory muscle paralysis. Tx via injection of specific type or trivalent horse antitoxin (to all who ate the meal) and consider mechanical ventilation to peclude respiratory failure. The specific toxin can be isolated form serum orthe source food. Adequare pressure cooking/autoclaind during canning kills spores. If you MUST eat suspect food boil it for 10 minutes. Refrigerate foods after cooking. There is no vaccine. Infant Botulism (AKA Floopy Baby Syndrome)- Mostly type A or B CB in 2-4 month olds. spores from some formulae, honey, dust, or soil are ingested and germinate, coloniing the colon where exotoxin gets absorbed (Infections and Intoxication). Presents with several days of constipation followed gy flaccid paralysis/floopy state, ptosis, dialated pupils, lethargy, disphagia, weak cry, respiratory difficulties (can be fatal) [in all a descending paralysis]. Presence of toxin in food or feces aids in Dx [cytopathic effect on culture cells or ELISA, or mouse injection, orgnaism culture is rare]. Tx via prolonges mechanical ventilation and symptomatic. No horse antitoxin to prevent anaphylaxis or lifelong hypersentisivity. Human is available for Infant botulism only! Antibiotics are not useful. Generally they recover but not always, one possible casue of SIDS. Wound Botulism- rare, usually in IV drug users or contaminated wounds. Pcnis useful to tx as is horse Ab (the only variety where antibiotics is prescribed). Symptoms are as described above. Botulinum toxin A (botox) or B (myoblock) have medical uses as well. Muscel spasms, strabismus, facial pain from trigemnal neuralgia etc. Also used cosmetically. Aerosolized spres may be among hte greatest bioterrism threats, whereas the most common is toxin added to food and drink. Beware and report b/l symmetrical descending paraysis with respiratory failure 1-5 days after eating the contaminant. CDC gives trivalent horse Antitoxin, and the DOD has heptavalent. Check for hypersensitivity first. |
|
|
Anaerobic Specimen Collection, transport, and ID
|
Collection-
blood culturs taken into anerobic media (blood bottles), aspirates of closed space infections, use syringe for collection (invasive procedure. Swabs are worst as they will pick up much normal flora. Inappropriate specimens to give meaningless into expectorated sputum ,throat swabs, nasal discharge, feces, vaginal swabs, voided urine, ileostomy/colostomy effluent an dbronchoscopy aspirates. Transport- rapid is important to rpevent overgrowth by facultatives. prefer liquid or tissue specimens, preferably bloody. Swabs as a last resort. Transport media for this purpose to retain unaltered proportans of viable organisms. Identification- based on morphology, gram stain, biochemical tests, and biochemical tests. can use gas-liquid chromatography etc...but gram staining is likely the most useful info for a quck clinicl decision. Card type tests are fast andsophisticated by expensive. Antibiotic sensitivity tests are not done as patterns are already established, so that we need to examine clues. We use the fast E-test for sensitivity of anaerobes if anything. |
|
|
family Mycoplasmataceae
|
contains two genera that infect humans: Mycoplasma and Ureaplasma, referred to collectively as mycoplasmas.
About 11 species are found in or on humans; many are part of normal oral flora. Only three species of mycoplasmas are recognized as human pathogens M. pneumoniae, M. Hominis, U. urealyticum. Smallest free-living microorganisms; diameter about 0.2-0.3 mm Can pass through some filters used to remove bacteria Lack a cell wall; bound by triple-layered membrane that, unlike other bacteria, contains sterols. Because mycoplasmas have no cell wall, are not susceptible to penicillin Sterols not synthesized by organism, essential for growth and membrane formation Highly plastic, can assume multiple shapes including round, pear shaped and filamentous Have the smallest genome size and, as a result, lack many metabolic pathways and require complex media for their isolation (circular genome, 1/5th size of E.coli) Grow slowly; center of colony grows into the agar and appears denser, giving appearance of an inverted “fried egg” Growth in culture inhibited by specific antisera directed at the particular species. Many mycoplasma species are known to be specific agents of diseases in animals. Causative agents are species specific. Bovine pleuropneumonia is oldest and best-known example and the old name, Pleuropneumonia-like organisms or PPLO, was derived from this disease. |
|
|
Mycoplasma Pneumoniae
|
Colonies of M. pneumoniae bind red blood cells (RBC) onto the surface of agar plate cultures (hemadsorbtion).
This is due to binding by the mycoplasma to sialic acid-containing oligosaccharides on surface of RBC Produces a common form of pneumonia (primary atypical “walking” or Eaton Agent pneumonia), whicho ccurs in any season, predilection for younger individuals, includes nonproductive cough, malaise, fever, headache, and scattered areas of pneumonia. It is usually benign. The disease is spread by close contact via aerosolized droplets Incubation period is relatively long (up to 3 weeks), prolonged shedding in nasal secretions may cause infections to be spread The mycoplasmas are extracellular pathogens that adhere to epithelial cell surfaces. Initially organism attaches to cilia and microvilli of cells lining the bronchial epithelium. Thus, adherence proteins are one of the major virulence factors. The adherence protein P1 localizes at tips of the bacterial cells and binds to oligosaccharides containing sialic acid residues on host epithelial cells. Mycoplasma produces adherence accessory proteins that also aid in adherence. Oligosaccharide receptors chemically similar to I antigen on surface of RBC. Not found on nonciliated goblet cells or mucus, to which M. pneumoniae does not bind. Colonization of the respiratory tract by M. pneumoniae results in the cessation of ciliary movement. The normal clearance mechanisms of the respiratory tract do not function, resulting in contamination of the respiratory tract and the development of a dry cough. Desquamation of involved mucosa and subsequent inflammatory reaction and exudate. The intimate association of the mycoplasma and the host cells allows transfer of toxic metabolic products (ROS). Furthermore, the mycoplasmas have been shown to inhibit host cell catalase. No true exotoxins isolated. In the early stages of infection diagnosis must be made on clinical grounds. 1. Microscopy - This useful in eliminating other possible pathogens. 2. Culture - Sputum (usually scant) or throat washings. Because of its slow growth, it may take 2 -3 weeks to get a positive identification, but culture is essential for a definitive diagnosis. Use agar medium that contains ascitic fluid or horse serum (enrichments), thallium acetate, penicillin and amphotericin B (inhibitors vs. other bacteria). Dx based on morphology, B hemolysis, and hemadsorption. 3. Serological tests are more commonly used for diagnosis. (Complement Fixatin, Cold Hemmaglutination [Ab's bind RBC I antigen at 4 degrees but not 37], ELISA.) Since mycoplasmas lack a cell wall, the penicillins and cephalosporins are ineffective. The antibiotics of choice are tetracycline (adults only) and erythromycin. Prevention is a problem due to the long duration of the disease. It is problematic to isolate patients to avoid close contact for a long period of time. No vaccines are currently available. |
|
|
Mycoplasma Hominis
|
Common inhabitant of genitourinary tract.
Best detected on Mycoplasma agar M. Hominis and Ureaplasma can be distinguished by arginine breakdown (M. hominis) and urease activitiy (U. urealyticum). Clinical syndromes - associated with pelvic inflammatory disease and post-partum fevers. Isolated from blood of about 10% of women with post-partum fevers. Pelvic inflammatory disease syndromes may be associated with M. hominis infection of fallopian tubes. Treatment: sensitive to tetracycline, resistant to erythromycin Colonization for both can occur during birth, in most cases the infection will be cleared. Only in a small number of cases does colonization persist. When individuals become sexually active, colonization rates increase. Approximately 15% are colonized with M. hominis and 45% - 75% with U. urealyticum. The carriers are asymptomatic but the organisms can be opportunistic pathogens. |
|
|
Ureaplasma Urealyticum
|
Distinguished from Mycoplasma by production of urease
On agar, colonies small, circular and grow downward. In liquid culture, using media containing urea and phenol red, growth results in production of ammonia from urea, increase in pH and change in color of indicator Colonies extremely small, thus Ureaplasma are also called T-strains (tiny strains). Manifestations: because of high colonization rate, difficult to associate specific illness with Ureaplasma One half of non-gonococcal urethritis in men Postpartum fever, organism isolated from 10% of women with condition Treatment: Men with urethritis treat with tetracyclin, also active against Chlamydia. Some tet resistent strains. Women, treat with tetracycline Colonization for both can occur during birth, in most cases the infection will be cleared. Only in a small number of cases does colonization persist. When individuals become sexually active, colonization rates increase. Approximately 15% are colonized with M. hominis and 45% - 75% with U. urealyticum. The carriers are asymptomatic but the organisms can be opportunistic pathogens. Colonization with U. urealyticum occurs in more than 80% of individuals who have had three or more sexual partners. |
|
|
L-Forms (Phase Varients)
|
L = lacking cell walls. They can be made or appear spontaneously, and form fried egg type colonies like mycoplasma. Highly pleiomorphic, derived form a wide variety from Gram +/- strains. They may be unstable and revert to bacterial forms, or may not revert. It is nearly impossible to derive the original strain of a stabile L form. Osmotic fragility is variable. L forms themselves are not usually infectious, however they can be induced in vivo allowing microbes to persist through host defense mechanisms/antibiotics. However to continue to be pathogenic, as far as we know for now, they need to revert.
|
|
|
Mycobacteria (shared characteristics)
|
Gram + slim rods, non-motile, non spore forming, obligate aerobes, slow growing. Cat + (with a few exceptions including INH resistant M. tuberculosis.
Cell Wall- instead of NAM it contains N-glycolylmuramic acid. They also contain mycolic acids (>60% of the total cell wall mass). Also has lipoarabinomannan extending form the plasma membrane. High lipid content makes the cell surface hydrophobic (difficult to stain, but resist decolarization (distinguishes it from other bacteria). They are pretty permiscuous in terms of growth requirements. Some nonpathogens can even grow on water faucits as opposed to M. Leprae which is an obligate intracellular parasite. Growth is slow as hydrophobiity causes clumping and limits permeability to nutrients (addition of surfactant/detergent increases growth rate). Diseases generally develop slowly, followed by a chronic course (granumolatous response). Infectivity is high, but virulence for healthy humans is low. They do not produce classic endo or exotoxins, the disease is a result of the host responses including delayed type hypersensitivity (DTH, destruction of non-activated macrophages containing organisms) and cell mediated immunity (CMI) that activates macrophages. |
|
|
Mycobacterium Tuberculosis
|
Gram + acid fast bacilli requiring rich growth medium @ 37 degrees. Aerobic with a doubling time of 18 hrs, colonies visable after 3-6 weeks. The cell wall structure contains high lipids and lipoarabinomannan (LAM, unique). It has many antigens including tuberculin (heat stable protein) which is the basis for Purified Protein Derivitive (PPD) testing. MT is resistant to drying, disinfectants, acids, and alkalis, but susceptable to UV, Heat, formaldehyde, and gluteraldehyde. Examine for large quantities of niacin, uncommon in other mycobacteria. Tuberculosis is still a major problem in the US (esp in urban poor areas) and world wide. There was a spike in the 80's as a result of AIDS (we are seeing more latent RB reativating, the liklyhood of which increases 2-3 times with HIV coinfection), Emigration, Drug resistance, and Crowding of homeless with poor nutrition. Today 1/3 of world has TB, all those infected are at risk for developing disease. Responsible for 6% of all deaths, 26% of preventable deaths. Multiple antibiotic resistance n endemic countries is 30-80%. Spreads from human to human via droplets (<10 bacilli may infect a susceptable individual). esp bad in closed environments.
Virulence: caused by one of 3 processes [ progression of primary infectin, exogenous reinfectionl or endogenous reavtivation]. Primary infection- generally a pulmonary development at the midzone fo the lung (bacilli taken all the way in are killed by mcriphages). some surviving bacilli enter non-activated blood macrophages and disseminate to hilar lymph nodes and then systemically. Signs and symptoms are often absent (The primay site of infection and node are often detected radiologially). The tubercle that results is a granuloma with activated multinucleated giant cells in the middle. When bacterial load is high, dying macrophages secrete ROS and RNS that lead to caseous necrosis. The infections tend to be handled well. The lesions heal and the bacteria die, but in some (well oxygenated tissues) bacilli remain viable (for months to decades) which can be reactivated if host defenses weaken. The calcified tubercles and draining lymph nodes produce/are the Ghon complex. Reactuvation: After primary infectin resolutoin, 5-10% of people develop reinfection. Usually is happens when immune resistance is lowered as a result of old age, alcoholism, malnutrition, corticosteroids, immunosupressants, or AIDS. Reactivation occurs at the apex of the lung leading to the most infections form of the disease (the lesions show spreading tubercles with numerous bacilli and large areas of caseous necrosis). When the necrosis involves the walls of small bronchi, necrotic material is discharged and a pulmonary cavity and bronchial spred results. Characterized by chronic fever (Macrophage derived TNF), weight loss, and productive cough. Immunity: Humans have high innate immunity to TB, aquired resistance depends on the generation of effective cell mediated response (accumulation of T cells and activated microbial macrophages). Without CMI we get diseae progression but with it we get casseous necrosis and cavity formation. CMI takes 2-6 weeks after primary infection. Delayed Type Hypersensitivity destroys inactivated macrophages and releases viable mycobacteria into necrotic area unsuitable for multiplication. CMI is incomplete and loss of DTH and CMI can occur over time and lead to re-infection/clinical TB. Disease: Primary TB is either asymptomatic or causes fever and malaise in most cases. Radiographs may reveal infiltrates or Ghon complexes. 5% of the time primary disease reacts into reactivation TB or miliary TB in which bacilli spread to 2ndary sites and form tubercles. Reactivation Tb: 10% of those recoveringfrom primary will get clinical disease sometime in their lifetime. In the Us it happens mostly to men over 50, but in other countries younger members of both sexes. Immunosupression accelerates the process. Symptoms: Persistant cough (universal), Fever, Night Sweats, Loss of weight, Constant tiredness, loss of apetite. X-rays detect infiltrates and cavities. Reactivation can rarely involve other organs. Pumonary TB takes 2-5 years to cause death. Dx- PPD, smears, PCT, cultures (VERY SLOW) examined for niacinproduction, homology of DNA, and Gas Chromatography. Tx- Extensive time perios (9 mos) using 2 or more antibotics. Rifampin/Isoniazid work on both extra and intracellular bugs, Pyrazinamide acts at pH found in cells, and Strepto is only effective agains extracellular pathogens. Isoniazid is also given prophylacticaly if there is evidence of active primary complex (x-ray), PPD, Child in contact with infectios case, or evidence of primary infection in immunocompromised pt's (but ca cause hepatitis in pt's over 35). You can also use the Bacille calmette-Guerin vacine in individuals who are PPD- (HIV or immunocompromised individuals are contraindicated). Note that it interferes with the skin test. |
|
|
Mycobacterium Leprae
|
Causes Leprosy in asia, africa, and latin america. Produces deformity in 30% if untreated. Acid-Fast bacillus that cannot be grown in artificial media or tissue culture (but can in mice footpads, armadillo, etc). Growth takes 12-14 days. Similar cell wasll components to other mycobacteria, except for Phenolic glycolipid (PGL-1) which is a unique mycoside on ML. It is a chronic granulomatous disease of peripheral nerves and sperficial tissues (nasal mucosa for instance). Many kinds of lesions ranging from anesthetic skin lesions to disfiguring facial ones. Social implications. Transmission via droplets from nasal secretions. Humans are the main reservoir, infectivity is very low and the incubation period is 2-7 yrsup to 40 years.
Virulence- multiplies inside of host cells (obligate intracellular parasite), preferentialy macrophages and schwann cells, although periheral sensory nerves may be involved (causing anesthesias). Few Leprae are seen in tuberculoid lesions. Lepromatous leprosy occurs in CMI deficient individuals. Immunity: CMI mediated, range of disease correlates with DTH responsiveness to lepromin (skin test antigen). Tuberculoid leprosy has minimal disease and + skin tests...Lepromatous cases have a progressive disease and negative skin tests. 2 main forms with some intermediates occur: Tuberculoid leprosy: development of large flattened plaques on face, trunk, and limbs. Lesions are anesthetic with peripheral nerve involvement. The disease is indolent, slow to progress and heal, but is non-contageous. Dx via clinical picture and biopsy. Tx with dapsone and rifampin for 6 months (the former blocks para-aminobenzoic acid metabolism) Lepromatous leprosy: occurs in CMi deficient individuals and pt's anergic to lepromin. lesions are densely infiltrated with bacilli that may reach the bloodstream. Damage can be severe with loss of nasal bones, septum, and digits. Dx via Ziehl-neelsen stained scrapings (many bacilli visualized). Tx with dapsone, rifampin, and clofazimime for at least 2 years early Dx and tx is importnat, chemoprophylaxis with sulonamides for children in close contact with lepromatous cases. |
|
|
PPD Results
|
tuberculin skin test, measures delayed hypersensitivity to tuberculoprotein (also known as protein purified derivative from Mycobacterium tuberculosis, PPD).
PPD is standardized, and activity expressed in tuberculin units (TU). Intradermal injection, read after 48-72 hours. Negative Reactions to PPD: a. Person was never infected b. Person infected and has become anergic, AIDS patients c. Test done too soon after primary infection d. Healthy person previously infected and lost hypersensitivity over time Positive reactions to PPD: a. Previously infected and cured b. Previously infected and latent organisms still present c. Active disease d. BCG vaccination e. Infected with highly cross-reactive mycobacteria |
|
|
Atypical Mycobacteria (M. Avium and M. Intracellulare)
|
They are realted acid-fast organisms that grow only slightly faster than TB. In non AIDS they almost never occur, but with AIDS they are major bacterial opportunists with multiple drug resistance causing systemic (vs. pulmonary) disease. The lesions are often lepromatous.
|
|
|
List the characteristics of chlamydia and describe the life cycle
|
Obligate intracellular gram - bacteria that exists in 2 forms, the metabolically inert infections Elementary body (EB), and the non-infectious metabolicaly active replicative body (RB). There are 3 species, trachomatis (2/3 biotypes [trachoma and LGV] are pathogenic in man), psittaci, and pneumoniae. The EB form is spherical with a DNA core, trilaminar cytoplasmic and outer membranes. The latter has a cysteine rich major protein linked to itself and 2 minor proteins via disulfide bridges, the reductin of which may cause change to the RB.
The life cycle has 4 stages: Attachment- Via Specific receptor-ligand binding, charge mediated, and hydrophobi interactions. The relative importance of each depends on the organism in question. Entry- Upon attachment EB is rapidly internalized by normally non-phagocytic cells (into a phagosome) Change to RB with growth and replication- Replicatin takes place in the chlamydial phagosome. By 8 hours post entry EB is now a larger, less rigid RB which is more permeable to ATP/nutrients. for 2 hours it undergoes binary fission Change to EB and release- at the end of 24 hours some of the RB's revert to EB's which the infected cell eventually releases (sometimes via lysis, others not). Antibody plays an important role in host defense. The interferons inhibit replication, and PMN's that phagoytose chlamydia render it non-infectious within an hour (although it survives well in activated macrophages). CMI is also activated by infected cells. Note that there are 15 serotypes of C. Trachomatis, and monoclonal antibodies raised against those major outer membrane proteins effectively neutralize them). |
|
|
List the various species of chlamydia, describe the diseases they cause and the laboratory tests used in their diagnosis
|
C. Trahomatis
Serotypes D-K; Urethritis- This is the most common of all chlamydial infections in men, and the most common STD. Often casues NGU/PGU (Non/Post gonococcal urethritis). NGU is typically less acute than gonorrhea, has sn incubation period of 10-14 days from exposure and can cause dysuria, discharge, and more rarely epididymitis. In women it is generally asymptomatic, however it has been associated with cervicitis, endometritis, and salpingitis (leading to infertility or ectopic pregnancy. 1/3 of neonates exposed during delivery develop conjunctivitis and 1/6 pneumonia. Otitis Media and bronchilitis are also possible. Dx- One needs first to get a specimen with the appropriate cell types (It is an OIP and does not grow on media). The most specific and expensive test is cell culture (2-3 day incubation on cell culture dish followed by fluorescent antibody visulaization), Faster tests include smears for direct antibody test, ELISA, DNA probing, and PCR (making urine sample viable) Tx: Therapy is warranted in individuals with sufficiently high risk including men with gonorrhea, NGU, or epididymitis, and their sexual contacts. Women with gonorrhea, cervicitis, or PID and their sexual contacts. (antichlamydial and antigonococcal tx can/should be concurrent). Fluoroquinolones (esp Ofloxacin) can treat both. For simple cases (lower genital/rectal) doxycycline or azithromycin is recommended (erythromycin, oflaxicin, and amoxicillin are alternatives). Complex infections (epididymitis, proctitis) systemic Tx with tetracycline is indicated. Women with PID (potentially mixed) should be Tx'd with combiantons of cephalosporin and doxycycline, or clindamycin with aminoglycoside. Infants can use oral erythromycin (as should pregnant infected women. Note, there should be no sex with former partners until regimen is complete. Chlamydia trachomatis Types A, B, and C: The greatest single cause of blindness in the world. Transmission is via contact with infected secretions, esp where sanitation and personal hygiene are poor. Onset is abrupt conjunctivitis followed with corneal vascularization and blindness. bacterial infections are quite common with Trachoma. Chlamydia Trachomatis L1, L2, and L3: Lymphogranuloma Venereum is an STD prevalent in the tropics (but not the US). 7-12 days post infection a painless papule appears in the genital area, which heals. 1-2 months later lymph nodes become tender and swolen. The enlarged nodes (buboes) may break open and drain, blockage can lead to elephantiasis and rectal strictures. Chlamydia Psittaci: Ornithosis is aquired via inhalation of dried bird feces (at risk are those who work with birds/poultry). Infectin is often asymptomatic or mild however a severe and fatal pneumonia can develop. Incubatin for 1-3 weeks followed by abrupt onset of fever, chills, and atypical pneumonia. Tetracycline is the drug of choice. Chlamydia Pneumoniae Present in about 10% of pneumonia, and 5% of bronchitis and sinusitis. 7/10 infections are mildly or A-symptomatic, however they are particularly common in AIDS. There may be a link to atherisclerosis/heart disease as a heart muscle alpha myosin heavy chain is homologous to the outer membrane proteins of chlamydia (causes autoimmune issue). Tx is with tetracycline, or alternatively with erythromycin, axithromycin, or a fluoroquinolone. |
|
|
List the drugs used to treat chlamydial infections and the conditions under which they are used
|
Chlamydia Trachomitus (D-K): Therapy is warranted in individuals with sufficiently high risk including men with gonorrhea, NGU, or epididymitis, and their sexual contacts. Women with gonorrhea, cervicitis, or PID and their sexual contacts. (antichlamydial and antigonococcal tx can/should be concurrent). Fluoroquinolones (esp Ofloxacin) can treat both. For simple cases (lower genital/rectal) doxycycline or azithromycin is recommended (erythromycin, oflaxicin, and amoxicillin are alternatives). Complex infections (epididymitis, proctitis) systemic Tx with tetracycline is indicated. Women with PID (potentially mixed) should be Tx'd with combiantons of cephalosporin and doxycycline, or clindamycin with aminoglycoside. Infants can use oral erythromycin (as should pregnant infected women. Note, there should be no sex with former partners until regimen is complete.
Chlamydia Psittaci- Tetracycline is the drug of choice. Chlamydia Pneumoniae- Tx is with tetracycline, or alternatively with erythromycin, axithromycin, or a fluoroquinolone. |
|
|
List the characteristics of rickettsia
|
Gram - coccobacilli arranged alone in pairs, or in strands. They are small obligate intrcellular bacteria that, with the exception of Q fever, spread via arthropod vectors. To replicate they attach to cells and induce phagocytosis. Escaping the phagosome via phospholipase activity, it replicates in the cytoplasm. An unusual ADP/ATP transpoart system is thought to be responsible for obligate intercellular status.
|
|
|
List the various species of rickettsia, describe the diseases they cause and the laboratory tests used in their diagnosis
|
Rickettsia Rickettsii-
Rocky Mountain Spotted Fever was first described in the west, but occures in many areas and most prevalently in the appalachian and atlantic coastal plain. Natural hosts are ticks, as well as the reservoir and vector. It infects many animals and also humans. The tick must bite and feed for at least 4 hours to cause transmission. Incubation for one week following which are fever, headache, maculopapular rash (first on the palms and soles, spreading to the trunk). Lesions in capillary endothelia lead to increased permeability causing peripheral and pulmonary edema. Fatality w/o antibiotics is about 25%, /w is 5-7%. Rickettsia Prowazekii- Epidemic typhus usually occurs with poor living conditions and sanitation. Humans are the primary reservoir, and transmit it via the body louse. They are excreted in the lice feces and then the skin is innoculated when we scratch the itch. Replication happens in the endothelial cells of blood vessels and there is a 6-15 day incubation period. Symptoms include fever, headache, chills, malaise, aches/pains, macular rash begining on the trunk ans spreading to the extremeties. In fatal cases death occurs in 2-3 weeks after onset. Brills disease, (milder form of typhus) results form reactivation of latent rickettsiae. Mortality similar to rocky mountain spotted fever. There is a living attenuated vaccine for epidemic typhus. One infected, long term antibiotics are indicated to eliminate residual latent organisms. Rickettsia Typhi- Endemic typhus(Murine typhus) resides in rats and, to a lesser degree, other rodents. It is transmitted from rat to rat by the rat flea, which is also how humans get it. After 1-2 weeks incubation we can expect fever, headache, and a macular rash. Rarely fatal. Coxiella Burnetii- Q fever is unique in that it does not require an arthropod vector to infect humans. It exists in a vegetative formthat replicates within cells, and also an endospore like form which is stabile outside and in heat. Tics still serve as the main reservoir, but they infect sheep and cattle. We get the infection by inhaling organisms shed by the animls. The incubation period is 2-3 weeks and is followed by fever, chills, headache, malaise, weakness, severe sweats, and often a pneumonis with mild cough. Mortality <1%. Q fever is treated wtih a combo of lincomycin and tetracycline in the long term to eliminate latent organisms. In general rickettsiae can be cultured in cells, animals, and the yolk sac of embryonated chicken eggs. They can then be stained and treated with fluorescent antibodies. More common though is serologial diagnosis via complement fixatin, indirect immunofluorescene, indirect hemagglutination, and latex agglutination. For Rocky Mountain Spotted Fever either PCR or indirect immunofluorescence with speciic antisera can be applied to skin biopsies from rash lesions. |
|
|
List the drugs used to treat rickettsial infections
|
There is a living attenuated vaccine for epidemic typhus. Prevention is often a matter of controling rodent reservoirs and the arthropod vectors. Chloramphenicol and Tetracycline are used to treat these infectins. Q fever is treated wtih a combo of lincomycin and tetracycline. In epidemic typhus and Q fever long term tx is indicated to eliminate residual latent infections.
|
|
|
Describe the infections caused by Ehrlichia and Bartonella henselae
|
Ehrlichia-
Transmitted by Ticks, these bugs cause disease similar to rocky mountasin spotted fever, except there is no rash. E. Chaffeensis casues human monocytic ehrlichiosis (HME) which infects monocytes/macrophages (primarily in SE and lower MW USA). Anaplasma/Ehrlichia phagocytophilum infects mostly neutrophils causing human granulocytic ehrlichiosis (HGE). This is the 2nd most common form of tick infection (beind lyme), and also distributed into the Northern USA by the same tick as lyme. They can be detected via serology and PCR, and can be controlled effectiely with doxycycline. Bartonella henselae- causes fever and adenitis associated with a distal papular lesion. Resides in the lymph nodes, and nearly 90% of those with suspected cat scratch disease have serological evidance. There are more than 20,000 cases in the US per year, and in one study B. Henselae was detected in 28 % of cats. Most cats carry the organism and are asymptomatic. |

