![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
160 Cards in this Set
- Front
- Back
|
Microbial Energetics: Why should you care? |
An understanding of how microbes obtain nutrients and energy is important if you want to grow them (or kill them) |
|
|
Define cell metabolism |
Metabolism is defined as the chemical reactions that occur within the cell to support basic life processes |
|
|
Name and define the two types of metabolic reactions |
1. Catabolic - release energy 2. Anabolic - use energy |
|
|
Define macronutrients |
Nutrients that are required in large amounts |
|
|
What are the two main macronutrients? |
The 2 main macronutrients are Carbon (C)and Nitrogen (N) |
|
|
Almost all macronutrients contain _____. |
Carbon |
|
|
A cell's dry weight is what percent carbon? |
50% |
|
|
What is nitrogen essential for? |
Nitrogen is essential for proteins and nucleic acids (RNA and DNA) |
|
|
Where is Nitrogen and Carbon usually obtained from |
N & C usually obtain from organic compounds (i.e. food sources) |
|
|
All organism get carbon from organic sources (T/F) |
False. Some prokaryotes can use inorganic carbon (CO2) as a carbon source (autotrophs) |
|
|
List off some of the other macronutrients |
1. Phosphorus (P) 2. Sulfur (S) 3. Potassium (K) 4. Magnesium (Mg) 5. Sodium (Na) 6. Calcium (Ca) 7. Iron (Fe) |
|
|
What is Phosphorus used for? |
nucleic acids and phospholipids |
|
|
What is sulfur used for? |
Amino acids and some vitamins |
|
|
What is Potassium used for? |
Enzymes involved in protein synthesis |
|
|
What is magnesium used for? |
Stabilizes ribosomes, cell membranes, nucleic acids, and for some enzyme activity |
|
|
What is sodium used to? |
Required by some microbes. Usually required by organisms that live in salty environments |
|
|
What is calcium used for? |
Not essential for all organisms. Helps stabilize the cell wall and involved in heat stability of endospores |
|
|
What is iron used for? |
Plays a major role in respiration. Cells produce siderophores to bind and transport it (bind Fe3+) |
|
|
What bacteria make entrobactins? |
E. Coli and Salmonella make entrobactins, complex siderophores |
|
|
Enterobactins have a high affinity for iron. (T/F) |
True |
|
|
Many disease causing organisms would survive in the host without enterobactins. (T/F) |
False. Many disease causing organisms would not survive in the host without these compounds |
|
|
What is iron sequestration a strategy for by animals? |
Iron sequestration is a strategy that may be used by animals to limit pathogens |
|
|
Define aquachelins |
Aquachelins - siderophores made by marine microorganisms |
|
|
What do aquachelins have a very high affinity for? |
Very high affinity for iron, since iron is low in seawater (picogram/ml) |
|
|
Aquachelins have a _____ tail associated with the cell membrane |
Hydrophobic |
|
|
What do aquachelins do? |
Aggregate and transport iron into the cell |
|
|
Define Free Energy (G) |
Is the energy in a chemical reaction that is available to do useful work |
|
|
Define free energy change (triange G) |
is the amount of energy released from a reaction that is available to do work |
|
|
What happens if triangle G is negative? |
The reaction releases energy (exergonic) |
|
|
Define exergonic |
If triangle G is negative - the reaction releases energy |
|
|
What happens if triangle G is positive? |
The reaction requires energy (Endergonic) |
|
|
Define Endergonic |
If triangle G is positive - the reaction requires energy |
|
|
What does triangle G knot tell us? |
Only tells us the energetics of the reaction |
|
|
What doesn't triangle G knot tell us? |
It doesn't tell us anything about the rate of the reaction |
|
|
Can reactions be slow? |
Yes, the rate may be very slow and in fact may take years |
|
|
Why might some reactions be very slow? |
This is because chemical bonds in the reactants need to be broken first in order to bring all of them into the reactive state |
|
|
What is needed to bring everything into the reactive state? |
This requires energy - Activation Energy |
|
|
How does a diamond transform from diamond to graphite |
The transformation of diamond to graphite has a negative triangle G knote so occurs spontaneously at STP but at a very, very, very slow rate! |
|
|
Activation Energy and Catalysis picture |
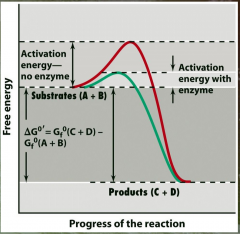
|
|
|
Define enzymes |
Catalytic proteins that speed up the rate of biochemical reactions by lowering the activation energy |
|
|
How do enzymes work? |
Temporarily bind to the substrate with noncovalent weak bonds - hydrogen bonds, van der Waals forces, etc. Alter the 3-D shape of the substrate, aligns reactive groups and weakens specific bonds Releases the products (s) and is recycled |
|
|
What is one of the fastest known enzymes? |
Among the fastest known enzymes is carbonic anhydrase, which catalyzes the reaction H2CO3 -->H20 + CO2. |
|
|
How fast can carbonic anhydrase make a reaction go? |
Can catalyze up to 10^6 reactions per second |
|
|
What is one of the slowest enzymes? |
Among the slowest is RuBisCo, which photosynthetic organisms use to "fix" atmospheric CO2 |
|
|
How fast does RuBisCo enzyme work? |
Catalyzes fewer than 10 reactions per second |
|
|
The energy of chemical reactions is conserved in biological systems. (T/F) |
True |
|
|
How is the energy of chemical reactions conserved in biological systems? |
This is accomplished by redox reactions |
|
|
What do redox reactions involve? |
Redox reactions involve the transfer of electrons from one reactant to another |
|
|
Define electron donor |
The reactant that gives the electrons is called the electron donor |
|
|
What happens when a reactant gives an electron? |
It is oxidized |
|
|
Oxidation does not involve oxygen. (T/F) |
False. Oxidation does not NECESSARILY involve oxygen |
|
|
Define electron acceptor |
The reactant that receives the electrons is called the electron acceptor |
|
|
What happens when a reactant receives an electron? |
It is reduced |
|
|
In chemistry you are reduced when you get electrons. (T/F) |
True |
|
|
How is H20 formed via redox? (picture) |

|
|
|
Define reduction potential |
The tendency of a compound to accept or release electrons is expressed quantitatively by its reduction potential |
|
|
What is a convenient way to very electron transfer potential? Why? |
A convenient way to view electron transfer potential is the Electron Tower, which gives the reduction potential for different redox couples |
|
|
How are redox couples written? |
Redox couples are written with the oxidized form of the couple on the left. |
|
|
What does a negative value indication with H2 |
The negative value indicates that H2 has a great tendency to donate electrons (become oxidized) than protons do to accept them. |
|
|
Where are the most negative redox couples located on the tower? |
The most negative redox couples (most likely to give up electrons) are at the top of the tower |
|
|
Where are the most positive redox couples located on the tower? |
The most positive couples (most likely to accept them) are at the bottom |
|
|
How does the electron tower work? |
Electrons donated from the top of the tower are "caught" by acceptors lower down the ladder |
|
|
What happens as an electron "falls" down the tower? |
The farther an electron "falls" down the tower, the more energy is released |
|
|
For every molecule of glucose respires, how many electrons travel down the respiratory chain? |
For every molecule of glucose respired, 24 electrons travel down the respiratory chain to the final acceptor: oxygen molecules |
|
|
How is the energy released in redox reactions conserved? |
The energy released in redox reactions is conserved in certain compounds that contain energy-rich phosphate or sulfur bonds |
|
|
What is the most common energy-rich phosphate bond? |
The most common of these compounds is adenosine triphosphate (ATP), the prime energy carrier in the cell |
|
|
What is long-term storage of energy linked to? |
Long-term storage of energy is linked to the formation of insoluble polymers (chains of repeated subunits), which can be consumed to yield ATP |
|
|
What are two polymers in prokaryotes? |
1. Glycogen 2. Poly- _ - hydroxybutyrate (PHB) |
|
|
What is Glycogen a polymer of? |
A polymer of glucose |
|
|
What is poly - _ - hydroxybutyrate (PHB) a polymer of? |
A polymer of lipids |
|
|
What are two polymers in Eukaryotes? |
1. Starch 2. Lipid storage |
|
|
What is starch a polymer of? |
A polymer of glucose |
|
|
How do eukaryotes conduct lipid storage? |
Lipid storage in the form of simple fats |
|
|
Define Catabolism |
Biochemical processes involved in the breakdown of organic/inorganic compounds, leading to the production of energy |
|
|
What are the 2 major types of catatbolic reactions in chemoorganotrophs? |
1. Fermentation 2. Respiration |
|
|
When does Fermentation occur? |
Occurs in the absence of O2 |
|
|
What does fermentation produce and how? |
Produces ATP by substrate level phosphorylation |
|
|
Fermentation produces more energy than respiration. (T/F) |
False. Produces less energy than respiration |
|
|
When does respiration occur? |
Can occur in the presence or absence of O2 |
|
|
How does respiration produce ATP |
ATP from oxidative phosphorylation - uses the proton motive force |
|
|
Respiration produces significantly more energy than fermentation. (T/F) |
True |
|
|
Define substrate-level phosphorylation |
Phosphate is added to an intermediate in the pathway and eventually transferred to ADP to make ATP |
|
|
What catabolic pathway does substrate-level phosphorylation occur in? |
Fermentation |
|
|
Define oxidative phosphorylation |
A proton motive force is set up across the cytoplasmic membrane and this energy source is used to make ATP |
|
|
What catabolic pathway does Oxidative phosphorylation occur? |
Respiration |
|
|
Define Glycolysis |
Glycolysis is the fermentation of glucose |
|
|
Like all fermentations, glycolysis, is _____/____ |
Internally balanced |
|
|
Define internally balanced |
Some atoms of the electron donor become reduced and other become oxidized |
|
|
What are the three stages of glycolysis and what happens in each stage? |
Stage 1 - Rearrangement & phosphorylation of substrate, no redox reactions Stage 2 - Redox reactions occur, ATP is produced Stage 3 - Redox reactions & formation of the products |
|
|
What is the first stage of glycolysis and what happens? |
Stage 1: Preparatory Reactions This step requires energy 2 molecules of ATP are used to make one molecule of fructose-1,6-bisphosphate Aldolase the splits fructose-1,6-bisphosphate into glyceraldehyde-3-P and dihydroxyacetone-3-P |
|
|
Does stage 1 of glycolysis require energy? If so how much? |
This step requires energy 2 molecules of ATP are used to make one molecule of fructose-1,6-biphosphate |
|
|
What is the name of stage two of glycolysis and what happens? |
First redox reaction -> NAD+ reduced to NADH and glyceraldehyde-3-P is phosphorylated to1,3-bisphosphoglycerate 2 ATPs formed with 1,3-bisphosphoglycerate converted to 3-P-glycerate 2 ATPs formed when phosphoenolypyruvateate converted to pyruvate 2 ATPs used in stage 1, net 2 ATPS |
|
|
What is the net ATPs by stage 2 of glycolysis? |
2 ATP |
|
|
What is the name of the third stage of glycolysis and what happens? |
Stage 3: Fermentation Products Pyruvate is converted to various fermentation products NADH is oxidized back to NAD+ during fermentation product formation Ethanol and CO2 produced by yeast Lactic acid produced by bacteria (and us!) Ultimate result of glycolysis is consumption of glucose, net synthesis of 2 ATPs, and fermentation products |
|
|
What is produced during the third stage of glycolysis? |
Ethanol, CO2, and Lactic acid |
|
|
In the end, what is the net synthesis in the third stage of glycolysis? |
2 ATPs, and fermentation products |
|
|
What yeast have humans taken advantage of? |
Humans have long taken advantage of fermentation in making bread, beer, and wine using the yeast Saccharomyces cerevisiae |
|
|
What is S. cerevisiae responsible for? |
S. cerevisiae is responsible for the alcohol fermentation of wines |
|
|
Why can S. cervisiae turn grapes into wine? |
Grape juice contains naturally high levels of sugars, which are converted into ethanol and CO2 |
|
|
Why can't you have a yeast concentration past 20%? |
The toxic effects of the ethanol on yeast prevent its concentration from exceeding 20% in most cases, but alcohol content may be increased through distillation |
|
|
Wine was probably produced accidentally as early as _____ years ago |
10,000 |
|
|
How is bread fermented? |
During the fermentation process of bread, sugar is converted into ethanol and CO2 by S. cerevisiae The CO2 forms bubbles which are trapped by the gluten of the wheat causes the bread to "rise" (aka leavening) Most of the ethanol will be evaporated by baking, but you can smell it during the leavening process |
|
|
In respiration the substrate is completely oxidized to what? |
CO2 and water |
|
|
In aerobic respiration what usually acts as the final electron acceptor |
O2 |
|
|
What happens when electrons "fall" further on the tower? |
Make more energy available, thus producing more ATP |
|
|
Respiration involves _____/_____/_____ in the membrane and the production of the ______/_____/_____(_____) |
Electron/transport/carriers Proton/motive/force (PMF) |
|
|
Define NADH dehydrogenases |
Membrane associated proteins Transfer hydrogen atoms |
|
|
Flavoproteins |
Flavin mononucleotide (FMN) and flavin-adenine dinucleotide (FAD) |
|
|
Define Iron-Sulfur proteins |
(non-heme iron proteins) Wide range of reduction potentials, can act as different points in the chain |
|
|
Cytochromes |
Proteins contain an iron-porphyin ring called heme |
|
|
What happens during electron transport during respiration? |
Electrons flow down the reduction potential tower, and finally reach O2 (terminal electron acceptor in aerobic respiration) |
|
|
Define Proton motive force (PMF) |
Energy from electron flow is used to pump protons across the membrane |
|
|
What does generating a proton motive force consist of? |
Consists of a series of membrane-associated carriers arranged from most negative to most positive reduction potential |
|
|
What do components of the proton motive force chain do? |
Components of the chain alternate between hydrogen atom carriers (protons + electrons) and electron only carriers |
|
|
A proton motive force (PMF) is generated across the ______ |
membrane |
|
|
What are features found in all electron transport chains |
Presence of membrane-associated electron carriers in order of increasingly more positive Alternation in the chain of electron only and electron plus proton carriers Generation of a proton motive force (PMF) as a result of charge separation across the membrane |
|
|
How can PMF be compared to a waterwheel? |
In a simple waterwheel or a massive hydroelectric dam energy is captured as water flows from a higher to lower energy state |
|
|
What is ATP synthase also known as? |
Also known as ATPase |
|
|
What are the two parts to ATP Synthase? |
Membrane bound Fknot Cytoplasmic head F1 |
|
|
ATP Synthase is the biggest biological motor (T/F) |
False. Smallest biological motor |
|
|
How mach H+ must pass through the ATPase to synthesize how much ATP? |
3-4 H+ must pass through the ATPase to synthesize 1 molecule of ATP |
|
|
ATP synthase picture + explanation |
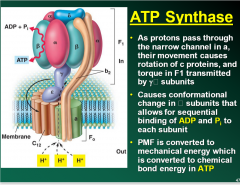
|
|
|
ATP Synthase can be ______ to generate the proton motive force if needed |
reversed |
|
|
What is the proton motive force also used for? |
Also used for substrate transport and motility |
|
|
ATPase also found in organisms that do not _____, used for _____/_____/_____/_____. |
Respire proton/motive/force/generation |
|
|
The "Chemiosmotic Hypothesis" was developed by who in what year? |
The "Chemiosmotic Hypothesis" was developed by Peter Mitchell in the 1960s |
|
|
What did Peter Mitchell propose in the Chemiosmotic Hypothesis? |
Mitchell proposed that protons could be moved across the membrane and this would represent the central energy currency in the cell |
|
|
In the Chemiosmotic Hypothesis how was energy conserved? |
In this hypothesis, energy would be conserved by a proton translocating ATP synthase |
|
|
When was Peter Mitchell award a Nobel Prize? |
The idea did not go over well at first, but eventually caught on; Mitchell was awarded the Novel Prize in Chemistry in 1978 |
|
|
Fermentation redox reactions are _____ balanced, without _____ electron acceptors |
balanced Exogenous |
|
|
Fermentation is Anaerobic or aerobic? |
Anaerobic |
|
|
Fermentation involves substrate-level phosphorylation (T/F) |
True |
|
|
In respiration redox reactions involves an exogenous electron acceptor (often oxygen) (T/F) |
True |
|
|
In respiration it is often anaerobic (but can be aerobic) (T/F) |
False. Often aerobic (but can be anaerobic) |
|
|
Where does respiration get energy? |
Energy from PMF via oxidative (electron transport) phosphorylation |
|
|
Where does the input (NADH/FADH) for electron transport come from in respiration? |
Substrate Catabolism During Respiration |
|
|
What is the first steps in substrate catabolism during respiration? |
The first steps in glucose metabolism by respiration are the same as during glycolysis - glycose is converted to pyruvate |
|
|
What happens to pyruvate during substrate catabolism during respiration |
Pyruvate is fully oxidized to CO2 by the Citric Acid Cycle (CAC), also known as the Tricarboxylic Acid Cycle or the Krebs Cycle |
|
|
What happens during the CAC in the substrate catabolism during respiration |
During the CAC electrons are passed into intermediates that are part of the electron transport chain |
|
|
Energy flow chart (picture) |
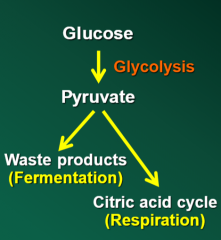
|
|
|
What happens before the transfer of electrons to the electron transport chain |
Pyruvate converted to CO2 and produces NADH, NADPH and FADH for transfer of electrons to the electron transport chain |
|
|
Electrons from NADH are not used to reduce pyruvate, but to feed electron transport chain (more energy) (T/F) |
True |
|
|
In the end, how much ATP is produced after glycolysis and CAC? |
38 ATP per glucose |
|
|
What happens during oxidative respiration In Mitochondria? |
Protons are pumped across this membrane as electrons flow through the respiratory chain |
|
|
What are other sources of energy production besides fermentation and aerobic respiration? |
Anaerobic Respiration Chemolithotrophy Phototrophy |
|
|
In anaerobic respiration what can function as terminal electron acceptors? |
In anaerobic respiration, electron acceptors other than O2 can function as terminal electron acceptors for energy generation |
|
|
What are other possible electron acceptors besides O2? |
Electron acceptors include nitrate (NO3-), ferric iron (Fe3+), and sulfate (S04[2-]) |
|
|
What happens when electron acceptors (besides O2) are higher on the electron tower? |
As these acceptors are higher on the electron tower, less energy is released than with oxygen, but allow respiration in low oxygen environments |
|
|
Define Chemolithotrophy |
Catabolism of inorganic compounds |
|
|
What are examples of inorganic electron donors? |
Hydrogen sulfide, hydrogen gas, ferrous iron |
|
|
What do Chemolithotrophy use to generate a proton motive force? |
Use an electron transport system to generate a proton motive force |
|
|
Many microorganisms can photosynthesize (T/F) |
True |
|
|
Define phototrophy |
Use light as energy to set up a proton motive force |
|
|
Define photoautotrophs |
Use CO2 as carbon source for biosynthetic reactions |
|
|
Define photoheterotrophs |
Use organic compounds as carbon sources for biosynthetic reactions |
|
|
What happened at the Chernobyl nuclear plant in 1986? |
On April 26, 1986 one of the reactors at the Chernobyl nuclear plant in Ukraine exploded, releasing large amounts of radioactivity over 100,000 km^2 and rendering some nearby areas uninhabitable by humans for least the next 300 years. But something has survived... |
|
|
What "survived" the Chernobyl explosion? |
Ionizing radiation changes the electronic properties of melanin and enhances the growth of melanized fungi |
|
|
Why is the PMF important? |
With the exception of fermentation, metabolism is respiration and photosynthesis revolves around electron transport and generation of a PMF |
|
|
Where can electrons come from in PMF |
Electrons can come from organic chemicals, inorganic chemicals, or from photosynthesis |
|
|
What does each electron involve? |
Each involves a membrane-associated electron transport chain, generation of a PMF, and synthesis of ATP by the ATPase |

