![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
103 Cards in this Set
- Front
- Back
|
identify components of enzymes
|
-encoded by genes.
|
|
|
identify fuction of enzymes
|
-biological catalyst; to speed up reactions at a temperature that is compatible with the normal functioning of the cell.
|
|
|
A substance that increases the rate of a chemical reaction but is not altered itself.
|
Catalyst
|
|
|
A molecule that catalyzes biochemical reactions in a living organism, usually a protein. An enzyme consisting of RNA that specifically acts on strands of RNA to remove introns and splice together the remaining exons.
|
Enzyme
|
|
|
Any compound with which an enzyme reacts.
|
Substrate
|
|
|
A temporary union of an enzyme and its substrate.
|
Enzyme–substrate complex
|
|
|
The protein portion of an enzyme, which requires activation by a coenzyme.
|
Apoenzyme
|
|
|
The nonprotein component of an enzyme.
|
Cofactor
|
|
|
A nonprotein substance that is associated with and that activates an enzyme.
|
Coenzyme
|
|
|
An enzyme consisting of an apoenzyme and a cofactor.
The Mechanism of Enzymatic Action |
Holoenzyme
|
|
|
Enzymes lower the activation energy of chemical reactions
|
The general sequence of events in enzyme action is as follows (Figure 5.4a):
The surface of the substrate contacts a specific region of the surface of the enzyme molecule called the active site. A temporary intermediate compound forms, called an enzyme–substrate complex. The substrate molecule is transformed by the rearrangement of existing atoms, the breakdown of the substrate molecule, or in combination with another substrate molecule. The transformed substrate molecules—the products of the reaction—are released from the enzyme molecule because they no longer fit in the active site of the enzyme. The unchanged enzyme is now free to react with other substrate molecules. As a result of these events, an enzyme speeds up a chemical reaction. |
|
|
Enzyme Components
|
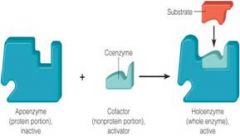
|
|
|
What are 6 factors influencing enzyme activity
|
•Temperature
•pH •substrate concentration •competitive inhibition •noncompetitive inhibition •feedback inhibition. |
|
|
For enzymatic reactions, however, elevation beyond a certain temperature (the optimal temperature) drastically reduces the rate of reaction.
The optimal temperature for most disease-producing bacteria in the human body is between 35°C and 40°C. |
temperature
|
|
|
A change in the molecular structure of a protein, usually making it
nonfunctional. Denaturation of a protein. Breakage of the noncovalent bonds (such as hydrogen bonds) that hold the active protein in its three-dimensional shape renders the denatured protein nonfunctional. |
denaturation
|
|
|
Most enzymes have an optimum pH at which their activity is characteristically maximal. Above or below this pH value, enzyme activity, and therefore the reaction rate, decline.
|
pH
|
|

The condition in which the active site on an enzyme is occupied by the substrate or product at all times.
|
Substrate Concentration - saturation
|
|
|
A chemical that competes with the normal substrate for the active site of an enzyme.
|
competitive inhibitor
|
|
|
An inhibitory chemical that does not compete with the substrate for an enzyme’s active site
|
noncompetitive inhibitor
|
|
|
The removal of electrons from a molecule.
|
oxidation
|
|
|
The addition of electrons to a molecule
|
reduction
|
|
|
An electron is transferred from molecule A to molecule B. In the process, molecule A is oxidized and molecule B is reduced.
|
oxidation-reduction
|
|
|
Much of the energy released during oxidation-reduction reactions is trapped within the cell by the formation of ATP. Specifically, a phosphate group, is added to ADP with the input of energy to form ATP.
|
The generation of ATP
|
|
|
The addition of a phosphate group to an organic molecule.
|
Phosphorylation
|
|
|
Most microorganisms oxidize carbohydrates as their primary source of cellular energy. Carbohydrate catabolism, the breakdown of carbohydrate molecules to produce energy, is therefore of great importance in cell metabolism. Glucose is the most common carbohydrate energy source used by cells.
|
Carbohydrate Catabolism
|
|
|
-2 ATP
-produces ATP and reduces NAD+ to NADH while oxidizing glucose to pyruvic acid. In respiration, the pyruvic acid is converted into the first reactant in |
Glycolysis
|
|
|
-4 ATP
-which produces ATP and reduces NAD+ (and another electron carrier called FADH2) while giving off CO2. The NADH from both processes carries electrons to |
Krebs cycle
|
|
|
-creates 34 ATP:
Prokaryotes: 38 ATP Eukaryotes: 36 ATP in which their energy is used to produce a great deal of ATP. In fermentation, the pyruvic acid and the electrons carried by NADH from glycolysis are incorporated into fermentation end-products. A small version of this figure will be included in figures throughout the chapter to indicate the relationships of different reactions to the overall processes. -NADH and FADH2 are oxidized, contributing the electrons they have carried from the substrates to a “cascade” of oxidation-reduction reactions involving a series of additional electron carriers. Energy from these reactions is used to generate a considerable amount of ATP. In respiration, most of the ATP is generated in the third step. |
(ETS) Electron transport chain
|
|
|
-final electron acceptor in ETC is oxygen. Krebs cycle.
-Respiration in which the final electron acceptor in the electron transport chain is molecular oxygen (O2). |
aerobic respiration
|
|
|
-final electron acceptor in ETC is NOT oxygen. Yields less energy than aerobic because only part of krebs cycle operates.
-Respiration in which the final electron acceptor in the electron transport chain is an inorganic molecule other than molecular oxygen (O2); for example, a nitrate ion or CO2. |
anaerobic respiration
|
|
|
final electron acceptor is organic molecule, ATP is synthesized, O2 is not required.
|
fermentation glucose
|
|
|
.
|
pyruvic acid
|
|
|
.
|
ATP
|
|
|
A series of redox reactions in a membrane that generates ATP; the final electron acceptor is usually an inorganic molecule.
|
cellular respiration
|
|
|
1. releases energy from sugars or other organic molecules, such as amino acids, organic acids, purines, and pyrimidines;
2. does not require oxygen (but sometimes can occur in its presence); 3. does not require the use of the Krebs cycle or an electron transport chain; 4. uses an organic molecule as the final electron acceptor; 5. produces only small amounts of ATP (only one or two ATP molecules for each molecule of starting material) because much of the original energy in glucose remains in the chemical bonds of the organic end-products, such as lactic acid or ethanol. |
fermentation
|
|
|
Comparison of Aerobic Respiration, Anaerobic Respiration and Fermentation
|
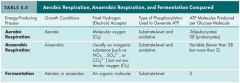
|
|
|
the sum of all the chemical reactions that occur in a living cell.
|
metabolism
|
|
|
synthesis reaction; The building of complex organic molecules to simple ones. Endergonic
|
anabolism
|
|
|
decomposition reaction; breakdown of complex organic compounds into simple ones. Exergonic.
|
catabolism
|
|
|
losing electrons
|
oxidation
|
|
|
gaining electrons.
|
reduction
|
|
|
intracellular energy source.
|
•ATP: adenosine triphosphate
|
|
|
the addition of a phosphate group to an organic molecule.
|
Phosphorylation
|
|
|
main pathway for the oxidation of glucose to pyruvic acid.
|
Glycolysis
|
|
|
converts to 2 carbon compounds to CO2, transferring electrons to NAD.
|
Krebs cycle
|
|
|
same as the krebs cycle.
|
Citric acid cycle
|
|
|
same as the citric acid cycle.
|
Tricarboxylic cycle (TCA)
|
|
|
a metabolic pathway that can occur simultaneously with glycolysis to produce pentoses and NADH without ATP production.
|
pentose phosphate pathway
|
|
|
uses light for energy source and CO2 for carbon source.
|
photoautotroph
|
|
|
light for energy source and organic compounds for carbon source.
|
Photoheterotroph
|
|
|
uses chemicals for energy source and CO2 for carbon source.
|
Chemoautotroph
|
|
|
uses chemicals for energy source and organic molecules for carbon source.
|
Chemoheterotroph
|
|
|
An organism that uses light at its primary energy source.
|
Phototroph
|
|
|
An organism that uses oxidation-reduction reactions as its primary energy source.
|
Chemotroph
|
|
|
An organism that uses carbon dioxide (CO2) as its principal carbon source. An organism that uses an inorganic chemical as an energy source and CO2 as a carbon source; An organism that uses light as its energy source and carbon dioxide (CO2) as its carbon source.
|
Autotroph
|
|
|
An organism that requires an organic carbon source; also called organotroph.
|
Heterotroph
|
|
|
hexose monophosphate shunt
|
pentose phosphate pathway
|
|
|
the final electron acceptor is oxygen
|
aerobic respiration
|
|
|
produces important intermediates that act as precursers in the synthesis of nucleic acids and so on.
|
pentose phosphate pathway
|
|
|
bacteria use oxygen subsitutes such as nitrates
|
anaerobic respiration
|
|
|
pyruvic acid accepts electrons and is turned into various end-products, such as lactic acid or ethanol
|
fermentation
|
|
|
The breakdown of carbohydrates to release energy
|
Glycolysis
Krebs cycle Electron transport chain |
|
|
glucose to pyruvic acid
|
glycolysis
|
|
|
electrons are removed from an organic compound and are transferred by an electron transport chain to oxygen
|
oxidative phosphorylation
|
|
|
an electron is liberated from chlorophyll and passes down an electron transport chain.
|
photophosphorylation
|
|
|
energy -yielding series of reactions
|
catabolism
|
|
|
means "whole enzyme"
|
holoenzyme
|
|
|
a non protein component of an active enzyme
|
coenzyme
|
|
|
a measure of the rate of activity of an enzyme
|
turnover number
|
|
|
a protein portion of an enzyme, inactive without a cofactor
|
apoenzyme
|
|
|
a group of enzymes that function as electron carries in respiration and photosynthesis
|
cytochromes
|
|
|
a mechanism by which fatty acids are degraded
|
beta oxidation
|
|
|
fermentation test
|
durham tube
|
|
|
both the carbon source and energy source are usually the same organic compound
|
chemoheterotroph
|
|
|
photosynthetic, but uses organic material rather than carbon dioxide as a carbon source
|
photoheterotroph
|
|
|
the photosynthetic purple nonsulfur bacteria would be classified in this nutritional group
|
photoheterotroph
|
|
|
photosynthetic bacteria that use carbon dioxide as a carbon source
|
photoautotroph
|
|
|
chagnes the shape of the active site of an enzyme
|
noncompetitive inhibitor
|
|
|
very similar in shape or chemistry to the normal enzyme substrate
|
competitive inhibitor
|
|
|
a dehydrogenase coenzyme dervied from nicotinic acid (niacin)
|
NAD*
|
|
|
a dehydrogenase coenzyme dervied from riboflavin
|
FMN
|
|
|
in chemiosmosis, protons can diffuse across a membrane only through special channels that contain this enzyme
|
ATP synthase
|
|
|
pyruvic acid loses carbon dioxide to form an acetyl group
|
decarboxylation
|
|
|
glycolysis
|
embden-meyerhof
|
|
|
a photosynthetic organism that does not produce oxygen
|
anoxygenic
|
|
|
removal of electrons
|
oxidation
|
|
|
uses an inorganic source of energy such as ammonia or elemental sulfur
|
chemoautotrophic
|
|
|
A chemoheterotroph that lives on dead organic matter
|
saprophyte
|
|
|
When an enzyme's active site is occupied at all times by substrate or product molecules
|
saturated
|
|
|
cyanide is an example of a general type of inhibitor called
|
noncompetitive
|
|
|
sulfa drugs are an example of a type of inhibitor called
|
competitive
|
|
|
in ______ phosphorylation, no oxygen or other inorganic final electron acceptor is required.
|
substrate-level
|
|
|
cyanobacteria produce _______ gas, just as do higher plants
|
oxygen
|
|
|
The amount of ATP yield from aerobic respiration by a prokaryote is ________
|
38
|
|
|
the amount of ATP yield from glycolysis is ________
|
2
|
|
|
The removal of NH2 from amino acid is called
|
deamination
|
|
|
the removal of -COOH from an amino acid is called
|
decarboxylation
|
|
|
the substance acted upon by an enzyme is called the
|
substrate
|
|
|
Coenzyme A is a derivative of the B vitamin _______ acid
|
pantothenic
|
|
|
A sequence of enzymatically catalyzed chemical reactions in a cell is called a ______ pathway
|
metabolic
|
|
|
Glucose is usually broken down to pyruvic acid by
|
glycolysis
|
|
|
In aerboic respiration, pyruvic acid is converted to acetyl ______: this product can then enter the Krebs Cycle
|
CoA
|
|
|
DNA and RNA are made up of repeating units called
|
nucleotides
|

