![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
97 Cards in this Set
- Front
- Back
|
What is the major storage form of glucose? |
Glycogen |
|
|
Where does glycogen exist? |
Liver and muscle |
|
|
What type of cells (specifically) require glucose? |
Cells with few to no mitochondria (red blood cells) |
|
|
What is the preferred energy source of the brain? |
Glucose |
|
|
What happens in animals if blood glucose levels fall? |
Glucagon is released if the a-cells sense the relative deficiency |
|
|
What does glucagon have to do with glucose? |
It affects its release from liver glycogen |
|
|
What happens in animals if glucose levels are high? |
Insulin is released from the beta cells in the pancreas |
|
|
What effect does insulin have on glucose? |
It affects glucose uptake by tissues (muscle and adipose) and it is needed for pancreatic a-cells to sense blood glucose levels |
|
|
What happens when pancreatic b-cells are destroyed? |
Insulin is no longer produced |
|
|
What happens when there is a high ratio of glycogen to insulin? |
Glycogen stores are broken down and depleted |
|
|
What is the structure of glycogen similar to? |
A-amylose |
|
|
How is glycogen broken down to glucose in the laboratory setting? |
Hydrolysis using a strong acid (ex. sulfuric acid) |
|
|
Purpose of experiment 1 |
Determine physiological effects of fasting and feeding on liver glycogen content |
|
|
How was diabetes induced? |
Injection of streptozotocin (destroys beta cells) |
|
|
What properties of glycogen were important in lab 1? |
The stability of its glycosidic bonds under alkaline conditions, its precipitation by ethanol and its hydrolysis under acidic conditions |
|
|
How was glycogen precipitated? |
The addition of ethanol |
|
|
Why did the liver need to be removed from the rat quickly? |
Prevent anaerobic glycogenolysis |
|
|
How was the liver homogenized? |
With KOH. |
|
|
What does a reagent blank do? |
Correct for color due to the reagent itself |
|
|
What does the oxygen electrode observe? |
1. The oxidation of substrates permeable to mitochondria 2. the effect of respiratory inhibitors on mitochondrial respiration |
|
|
How does the oxygen electrode observe oxidation? |
It obseves substrate oxidation by measuring the depletion of oxygen at the platinum electrode. |
|
|
Where are substrates oxidized? |
In the mitochondria. |
|
|
How does biological oxidation occur? |
Addition of oxygen, removal of hydrogen, removal of oxygens |
|
|
What inhibitors were used in experiment 2? |
Sodium azide, antimycin A, rotenone. |
|
|
Why was NaOH used in experiment 2? |
Neutralize glucose solutions. |
|
|
What substrates were used in experiment 2? |
Succinate and B-hydroxybutyrate |
|
|
How did sodium azide affect the substrates in experiment 2? |
Partially inhibited them |
|
|
How did antimycin A affect the substrates in experiment 2? |
Inhibited them |
|
|
How did rotenone affect the substrates in experiment 2? |
Inhibited B-hydroxybutyrate but not succinate |
|
|
What does the change in osmolarity in experiment 1 indicate? |
One of the advantages of storing glucose as glycogen is that it permits mobilization of glucose without gross alterations in the solute content (and hence osmotic pressure) of the liver cell. |
|
|
What does the oxygen electrode's specificity depend on? |
The diffusion of oxygen through a membrane that prevents metal ions (or other reducible species) from diffusing to the electrode |
|
|
How is the removal of H2 from metabolites mediated in experiment 2? |
Cofactors/electron carriers--pyridine/flavonucleotides |
|
|
Where does rotenone enter the respiratory chain? |
Complex 1 |
|
|
Where does Antimycin A enter the respiratory chain? |
Complex III |
|
|
Where do sodium azide and cyanide enter the respiratory chain? |
Complex 4 |
|
|
What does it mean if an inhibitor blocks one substrate but not another? |
It acts before the second complex or in one of the branches of the chain |
|
|
What does it mean if an inhibitor blocks both sustrates? |
It is acting in the common path of the chain |
|
|
What does EGTA chelate? |
Ca^2+ ions |
|
|
What is the major component of respiration medium? |
KCl |
|
|
What is in respiration medium? |
KCl, buffer, chelating agent (EDTA), bovine serum albumin
|
|
|
What is the pH of respiration medium? |
7.2 |
|
|
How is a polarograph interpreted? |
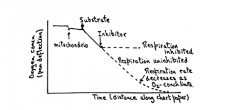
|
|
|
How are P:O ratios interpreted? |
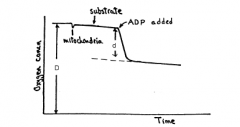
|
|
|
What does a small pen deflection on the polarograph result from? |
The fact that the polarograph is not temperature equilibriated. |
|
|
How does the Clark electrode work? |
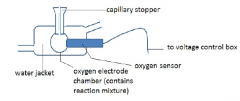
Platinum electrode and silver electrode are in contact with KCl but are separated by a thin Teflon membrane held in place by an O-ring.
The platinum electrode gets a negative charge relative to the silver electrode.
The sensor is placed in a water jacketed chamber so the sample and sensor can be |
|
|
What is the Teflon membrane permeable to? |
Molecular oxygen. |
|
|
How does the electrical current flow in the oxygen electrode? |
Oxygen goes through the Teflon membrane where it reaches the platinum electrode and is reduced to water. The current flows from the silver electrode to the platinum electrode as electrodes are removed. Silver ions combine with chloride to form silver chloride.
|
|
|
What reaction coupling speeds up the oxidation of NADH and FAD? |
ADP/Pi |
|
|
Why aren't NADH and FADH2 oxidized when added to mitochondria? |
They cannot permeate the inner membrane and get to their oxidation sites. |
|
|
What permeates the inner mitochondrial membrane in experiment 2? |
B-hydroxybutyrate and succinate |
|
|
Once NADH and FADH2 permeate the inner membrane what are they oxidized by? |
NAD+ and FAD |
|
|
What determines permeation? |
Presence of transport systems (NOT molecular size) |
|
|
How is the physiological link between electron transport and ATP synthesis uncoupled? |
Disruption of the inner mitochondrial membrane or by the uptake of Ca2+. |
|
|
When is EGTA used? |
As a chelating agent to remove Ca2+ during isolation |
|
|
When is EDTA used? |
As a chelating agent to remove Ca2+ in respiration medium |
|
|
What substrates cannot permeate the inner membrane? |
NAD, NADH, FAD2, FADH2, FMN AND FMNH |
|
|
What is electron transport physiologically coupled to? |
Oxidative phosphorylation |
|
|
What is needed to rapid oxidation to occur? |
ADP and inorganic phosphate |
|
|
What inhibitor was used in experiment 3? |
Oligomycin |
|
|
What does oligomycin inhibit? |
ATP synthase/respiration |
|
|
What uncoupler is used in experiment 3? |
2,4-dinitrophenol |
|
|
What does 2,4-DNP uncouple? |
Oxidative phosphorylation |
|
|
What did the polarograph measure in experiment 3? |
Diffusion current--proportional to the oxygen concentration of the solution in the |
|
|
What could happen if the respiration medium is shaken too vigourously? |
The albumin could denature and cause froth |
|
|
What does the ADP/O ratio measure? |
the ratio of the number of moles of phosphorus atoms incorporated into "high energy bonds" in ATP to the number of moles of oxygen atoms utilized or: number of ATPs produced per pair of electrons traveling through the electron transport system (ETS). |
|
|
In experiment 3, what do the moles of ADP measure? |
Phosphorylation |
|
|
In experiment 3, what does the decrease in concentration of O2 measure? |
Respiration rate |
|
|
What signifies a new rate of oxygen consumption on a polarograph chart? |
A plateau or a change of slope to that of the "background" apparent oxygen uptake. |
|
|
What are uncouplers? |
lipid soluble weak acids that uncouple respiratory chain activity from ATP synthesis |
|
|
How does oligomycin work? |
Oligomycin inhibits oxidative phosphorylation by inhibiting ATP synthase activity. Oligomycin blocks proton conduction through the F0 portion of the ATP synthase, preventing the synthesis of ATP |
|
|
What is required for oxidative phosphorylation? |
An intact inner mitochondrial membrane |
|
|
What is oxidative phosphorylation needed for? |
ATP synthesis |
|
|
What prevented ATP synthesis in experiment 3? |
Oligomycin |
|
|
Where does oligomycin bind in the ETC? |
Complex 5 |
|
|
What is the advantage of using isolated cells over sliced cells? |
Adequate oxygenation and ease of pipetting |
|
|
What is K-H saline gassed with? |
95% O2, 5% CO2 |
|
|
What controls the pH in experiment 4? |
CO2 |
|
|
What substrates were used in experiment 4? |
Na pyruvate, alanine, leucine |
|
|
How does the OsMolar scale work? |
1 M of any species in solution exerts |
|
|
What is the osmolarity of human blood plasma? |
About 300mOsM |
|
|
Why didn't we need to correct for dilution on the standard curve in experiment 4 as in experiment 1? |
The same amount of sample was used in the experimental and the standards |
|
|
What did it mean in experiment 4 if the solution was light pink to orange? |
Too acidic |
|
|
What did it mean in experiment 4 if the solution was purple or blue? |
Too basic |
|
|
What was used for back titration in experiment 4? |
Perchloric acid |
|
|
How was the rate of gluconeogenesis calculated in experiment 4? |
u moles glucose produced/min/mg dry wt of cells |
|
|
Why were the rats fasted in experiment 4? |
Because gluconeogenesis occurs in times of starvation--in the liver glucose is made from amino acids or glycerol in times of starvation |
|
|
Why is K-H saline used? |
It is a physiological saline and thus prevents osmotic damage to the blood cells |
|
|
Why is leucine not gluconeogenic? |
Because it converts to acetyl-CoA which will be given to the Kreb's Cycle to make L-glucose |
|
|
Why did Na Pyruvate and Alanine work in experiment 4? |
Because they convert to oxaloacetate |
|
|
What is Friedwald's equation? |
LDLcholesterol=Total cholesterol - HDL cholesterol - (triglyceride/2.2)
|
|
|
What is the reaction of cholesterol assay? |
cholesterol esters ---CEH-----> cholesterol + fatty acids
cholesterol + O2 -----------CO-------> cholest-4-en-3-one + H2O2 |
|
|
What is the reaction of triglyceride assay? |
Triglycerides + H2O (Lipase)---> Glycerol + Fatty Acids |
|
|
How is mg/dl converted to mM? |
18 × mmol/l
Here, use the molar mass of cholesterol, 386.6 g/mol (therefore, 386.6 mg/mmol) |
|
|
What SI unit is used for cholesterol? |
mM |
|
|
What SI unit is used for triglycerides? |
mg/dL |
|
|
What levels of serum cholesterol is considered elevated? |
5.2mM |
|
|
What levels of serum triglyceride is considered elevated? |
150 mg/dL |

