![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
61 Cards in this Set
- Front
- Back
|
activation energy
|
• The energy required to initiate a chemical reaction; also defined as the energy required to overcome the energy barrier to a chemical reaction
• Often supplied in the form of thermal energy (heat) that the reactant molecules absorb • Provides a barrier that determines the rate of the reaction • Absorption of thermal energy accelerates the reactant molecules, so they collide more often and more forcefully • It also agitates the atoms within the molecules, making the breakage of bonds more likely • When enough energy has been absorbed for the bonds to break, the reactants are in an unstable condition known as the transition state |
|
|
anabolic reactions
|
• Sometimes called biosynthetic pathways
• Metabolic reactions that synthesize larger molecules from smaller ones • Includes synthesis of an amino acid from simpler molecules and the synthesis of a protein from amino acids • Energy released from the downhill reactions of catabolic pathways can be stored and then used to drive the uphill reactions of anabolic pathways |
|
|
catabolic reactions
|
• Metabolic reactions that break down larger molecules into smaller ones
• A major pathway includes cellular respiration, in which the sugar glucose and other organic fuels are broken down in the presence of oxygen to carbon dioxide and water • Energy released from the downhill reactions of catabolic pathways can be stored and then used to drive the uphill reactions of anabolic pathways |
|
|
catalyst
|
A substance that lowers the activation energy of a chemical reaction without being itself consumed by the reaction
|
|
|
chemical energy
|
A form of potential energy contained in the chemical bonds between atoms of a molecule; refers to the potential energy available for release in a bond
|
|
|
endergonic
|
• Describing reactions that are non-spontaneous
• Describing a chemical reaction that requires an input of energy into the system • The products have higher free energy than the reactants • This kind of reaction essentially stores free energy in molecules (G increases), so ∆G is positive • Magnitude of ∆G is the quantity of energy required to drive the reaction • If a chemical process is exergonic (downhill), releasing energy in one direction, then the reverse process must be endergonic (uphill), using energy |
|
|
energy
|
The capacity to cause change, especially to do work (to move matter against an opposing force)
|
|
|
energy-coupling
|
• The linkage of an energy-releasing (exergonic) reaction to an energy-costing (endergonic) reaction to drive the energetically unfavorable endergonic reaction
• Coupled reactions must have an overall release of free energy in order to occur spontaneously • ATP is responsible for mediation of most energy coupling in cells, and in most cases acts as the immediate source of energy that powers cellular work |
|
|
entropy
|
• A measure of chaos or disorder in a system (randomness)
• In a particular system, such as an organism, entropy may actually decrease as long as the total entropy of the universe (the system plus its surroundings), increases |
|
|
enzyme
|
• Word first used by German physiologist Wilhelm Kühne in 1877 to describe the action of yeast leavening bread
• A macromolecule serving as a biological catalyst, a chemical agent that increases the rate of a reaction without being consumed by the reaction • Most enzymes are proteins |
|
|
exergonic
|
• Describing reactions that occur spontaneously
• Describing a chemical reaction with a net release of energy • Because the chemical mixture loses free energy (G decreases), ∆G is negative • Magnitude of ∆G for an exergonic reaction represents the maximum amount of work the reaction can perform • The greater the decrease in free energy, the greater the amount of work that can be done • If a chemical process is exergonic (downhill), releasing energy in one direction, then the reverse process must be endergonic (uphill), using energy |
|
|
first law of thermodynamics
|
• Also known as the principle of conservation of energy
• States that energy can be transformed from one form to another but cannot be created or destroyed; the amount of energy in a closed system is constant |
|
|
Gibbs free energy
|
The energy that can be used by a system to perform work when temperature and pressure are uniform; also called free energy
|
|
|
kinetic energy
|
• The energy associated with the relative motion of objects
• Moving matter can perform work by imparting motion to other matter |
|
|
metabolic pathway
|
A series of chemical reactions in a biological system in which molecules are assembled, decomposed or interconverted
|
|
|
metabolism
|
• The total chemical activity of a living organism; composed of both anabolic and catabolic reactions, which manage the material and energy resources of
the organism • An emergent property of life that arises from orderly interactions between molecules |
|
|
non-spontaneous reaction
|
• A reaction in which ∆G has a value greater than zero
• The reaction requires the addition of energy from the environment into the system |
|
|
potential energy
|
• The form of energy associated with the position or arrangement of objects in a system
• Molecules possess energy because of the arrangement of electrons in the bonds between their atoms |
|
|
second law of thermodynamics
|
• States that no energy transformation is perfectly efficient; in a closed system, some energy from every transformation will always be lost as heat, with a corresponding increase in entropy
• A system can put heat to work only when there is a temperature difference that results in the heat flowing from a warmer location to a cooler one |
|
|
spontaneous reaction
|
• A reaction in which ∆G has a value less than zero
• The reaction can occur without adding energy from the environment into the system • The process is energetically favorable • For a process to occur spontaneously, it must increase the entropy of the universe |
|
|
the energy of life
|
• Sugars can be converted to amino acids that are linked together into proteins when needed
• When food is digested, proteins are dismantled into amino acids that can be converted to sugars • Small molecules are assembled into polymers, which may be hydrolyzed later as the needs of the cell change • In multicellular organisms, many cells export chemical products that are used in other parts of the organism • The process called cellular respiration drives the cellular economy by extracting the energy stored in sugars and other fuels |
|
|
bioenergetics
|
1) The overall flow and transformation
of energy in an organism 2) The study of how energy flows through organisms |
|
|
thermal energy (heat)
|
• The total amount of kinetic energy due to the random motion of atoms or molecules in a body of matter
• Energy in its most random form |
|
|
thermodynamics
|
The study of energy transformations that occur in a collection of matter
|
|
|
system and surroundings
|
• The system denotes the matter under study
• The rest of the universe, or everything outside of the system, is known as the surroundings |
|
|
types of systems
|
• An isolated system, such as that approximated by the liquid in a thermos bottle, is unable to exchange either energy or matter with its surroundings
• In an open system, energy and matter can transferred between the system and its surroundings (ex. organisms -- they absorb energy such as light or chemical energy in the form of organic molecules and release heat and metabolic waste products, such as carbon dioxide, to the surroundings) |
|
|
change in free energy, ∆G with enthalpy, temperature, and entropy
|
• ∆G = ∆H - T∆S
• Where ∆H symbolizes the change in the system's enthalpy (in biological systems, equivalent to total energy), ∆S is the change in the system's entropy, and T is the absolute temperature in units of Kelvin • Only processes with a negative ∆G are spontaneous • For ∆G to be negative, either ∆H must be negative (the system gives up enthalpy and H decreases) or T∆S must be positive (the system gives up order and S increases), or both • In other words, all spontaneous processes decrease the system's free energy |
|
|
change in free energy, ∆G between final and initial states
|
• ∆G = ∆G(final) - ∆G(initial)
• ∆G can be negative only when the process involves a loss of free energy during the change from initial state to final state • So, change in free energy is a measure of a system's instability -- its tendency to change to a more stable state • Unstable systems (higher G) tend to change in such a way that they become more stable (lower G) |
|
|
equilibrium
|
• A state of maximum stability
• Most chemical reactions are reversible and proceed to a point at which the forward and backward reactions occur at the same rate, at which the reaction is then said to be at equilibrium, and there is no further net change in relative concentration of products and reactants • As a reaction proceeds toward equilibrium, the free energy of the mixture of reactants and products decreases • Any change from the equilibrium position will have a positive ∆G and will not be spontaneous; for this reason, systems never spontaneously move away from equilibrium • A process is spontaneous and can perform work only when it is moving toward equilibrium |
|
|
forming & breaking of bonds
|
• Breaking of bonds does not release energy; it requires energy
• Forming of bonds release energy |
|
|
metabolism and equilibrium
|
• As a whole, metabolism is never at equilibrium, which is one of the defining features of life (ex. the constant flow of materials in and out of a living cell keeps the metabolic pathways from ever reaching equilibrium, and the cell continues to do work throughout its life)
• The key to maintaining lack of equilibrium is that the product of a reaction does not accumulate but instead becomes a reaction in the next step; finally, waste products are expelled from the cell |
|
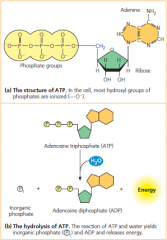
ATP (adenosine triphosphate) & ATP hydrolysis
|
• Nucleoside triphosphate that contains the sugar ribose, with the nitrogenous base adenine and a chain of three phosphate groups bonded to it
• Releases free energy when its phosphate bonds are hydrolyzed, which is used to drive endergonic reactions in cells |
|
|
types of work performed by cells
|
• Chemical work, the pushing of endergonic reactions that would not occur spontaneously, such as the synthesis of polymers from monomers
• Transport work, the pumping of substances across membranes against the direction of spontaneous movement • Mechanical work, such as the beating of cilia, the contraction of muscle cells, and the movement of chromosomes during cellular reproduction |
|
|
phosphorylated intermediate
|
A molecule (often a reactant) with a phosphate group covalently bound to it, making it more reactive (less stable) than the unphosphorylated molecule
|
|
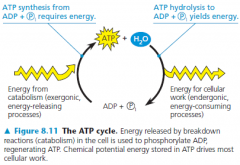
the regeneration of ATP
|
• ATP is a renewable resource that can be regenerated by the addition of a phosphate to ADP
• The regeneration of ATP from ADP and inorganic phosphate is endergonic |
|
|
the activation energy barrier
|
• Changing one molecule into another generally involves contorting the starting molecule into a highly unstable state before the reaction can proceed
• To reach the contorted state when bonds can change, reactant molecules must absorb energy from their surroundings • When the new bonds of the product molecules form, energy is released as heat, and the molecules return to stable shapes with lower energy than the contorted state |
|
|
substrate
|
• The reactant on which an enzyme works
• The enzyme binds to its substrate(s), forming an enzyme-substrate complex • Catalytic action of the enzyme converts the substrate to the product(s) of the reaction |
|
|
enzyme-substrate complex
|
• A temporary complex formed when an enzyme binds to its substrate molecule(s)
• Covalent bonds NOT typically used to stabilize enzyme-substrate complex |
|
|
enzyme specificity
|
• Most enzyme are proteins, which have unique three-dimensional configurations
• The specificity of an enzyme results from its shape, which is a consequence of its amino acid sequence • Only a restricted region of the enzyme molecule actually binds to the substrate |
|
|
active site
|
• The specific region of an enzyme that
binds the substrate and that forms the pocket in which catalysis occurs • Usually formed by only a few of the enzyme's amino acids, with the rest of the protein molecule providing a framework that determines the configuration of the active site • The specificity of an enzyme is attributed to a compatible fit between the shape of its active site and the shape of the substrate • Not a rigid receptacle for the substrate |
|
|
induced fit
|
• Caused by entry of the substrate, the change in shape of the active site of an enzyme so that it binds more snugly to the substrate
• Brings chemical groups of the active site into positions that enhance their ability to catalyze the chemical reaction |
|
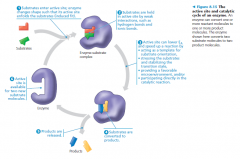
the catalytic cycle of an enzyme
|
• The rate at which a particular amount of enzyme converts substrate to product is partly a function of the initial concentration: the more substrate molecules that are available, and the more frequently they access the active sites of the enzyme molecules
• When the concentration of the substrate is high enough that all enzyme molecules have their active sites engaged, the enzyme is said to be saturated, and the rate of the reaction is determined by the speed at which the active site converts substrate to product • When an enzyme is saturated, the only way to increase the rate of product is to add more enzyme |
|
|
effects of temperature on enzyme activity
|
• Reactions increase with increasing temperature, partly because substrates collide with active sites more frequently when the molecules move rapidly
• Above that temperature, however, the speed of the reaction drops sharply • The thermal agitation of the enzyme molecule disrupts the hydrogen bonds, ionic bonds, and other weak interactions that stabilize the active shape of the enzyme, and the protein molecule eventually denatures • Each enzyme has an optimal temperature at which its reaction rate is greatest • Most human enzymes have optimal temperatures of about 35-40 degrees C (close to human body temperature) • Thermophilic bacteria that live in hot springs contain enzymes with optimal temperatures of 70 degrees C or higher |
|
|
effects of pH on enzyme activity
|
• The optimal pH values for most enzymes fall in the range of pH 6-8
• One exception is pepsin, a digestive enzyme in the human stomach, which works best at pH 2 • In constrast, trypsin, a digestive enzyme residing in the alkaline environment of the human intestine, which has an optimal pH of 8, would denature in the acidic environment of the stomach |
|
|
cofactors
|
• Any nonprotein molecule or ion that is required for the proper functioning of an enzyme
• Cofactors can be permanently bound to the active site or may bind loosely and reversibly, along with the substrate, during catalysis • Some cofactors of enzymes are inorganic such as metal atoms zinc, iron, and copper in ionic form • If the cofactor is an organic molecule, it is specifically called a coenzyme |
|
|
coenzyme
|
• An organic molecule serving as a cofactor
• Most vitamins function as coenzymes in metabolic reactions |
|
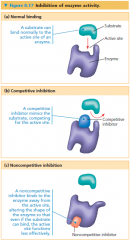
enzyme inhibitors
|
• If the inhibitor attaches to the enzyme by covalent bonds, inhibition is usually irreversible
• Many inhibitors bind to the enzyme by weak interactions, making the inhibition reversible • Competitive inhibitors resemble the normal substrate molecule and compete for admission into the active site • Noncompetitive inhibitors impede enzymatic reactions by binding to another part of the enzyme (they do not compete with the substrate to bind to the active site) |
|
|
competitive inhibitor
|
• A substance that reduces the activity of an enzyme by entering the active site in place of the substrate, whose structure it mimics
• May be overcome by increasing the concentration of substrate so that as active sites become available, more substrate molecules than inhibitor molecules are around to gain entry to the sites |
|
|
noncompetitive inhibitor
|
A substance that reduces the activity of an enzyme by binding to a location remote from the active site, changing the enzyme’s shape so that the active site no longer effectively catalyzes the conversion of substrate to product
|
|
|
the evolution of enzymes
|
• More than 4,000 different enzymes have been found in different species
• If the changed amino acids are in the active site or some other crucial region, the altered enzyme might have a novel activity or might bind to a different substrate • Under environmental conditions where the new function benefits the organism, natural selection would tend to favor the mutated form of the gene, causing it to persist in the population |
|
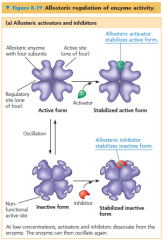
allosteric regulation
|
• The binding of a regulatory molecule to a protein at one site that affects the function of the protein at a different site
• May result in either inhibition or stimulation of an enzyme's activity |
|
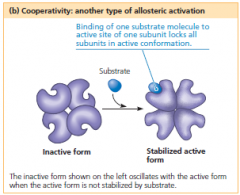
cooperativity
|
A kind of allosteric regulation whereby a shape change in one subunit of a protein caused by substrate binding is transmitted to all the other subunits, facilitating binding of additional substrate molecules to those subunits
|
|
|
allosteric activation and inhibition
|
• Most enzymes known to be allosterically regulated are constructed from two or more subunites, each composed of a polypeptide chain with its own active site
• Entire complex oscillates between two different shapes, one catalytically active and the other • An activating or inhibiting regulatory molecule binds to a regulatory site, often located where subunits join • The binding of an activator to a regulatory site stabilizes the shape that has functional active sites, whereas the binding of an inhibitor stabilizes the inactive form of the enzyme • A single activator or inhibitor molecule that binds to one regulatory site will affect the active sites of all subunits |
|
|
ATP & APD in allosteric activation & inhibition
|
• ATP binds to several catabolic enzymes allosterically, lowering their affinity for substrate and thus inhibiting their activity
• ADP however, functions as an activator of the same enzymes • If ATP production lags behind its use, ADP accumulates and activates the enzymes that speed up catabolism, producing more ATP • If the supply of ATP exceeds demand, then catabolism slows down as ATP molecules accumulate and bind to the same enzymes, inhibiting them |
|
|
identification of allosteric regulators
|
• Hard to characterize, in part because they tend to bind to the enzyme at low affinity and are therefore hard to isolate
• Allosteric regulators are attractive drug candidates because they exhibit higher specificity for particular enzymes than do inhibitors |
|
|
caspases
|
• Protein-digesting enzymes that play an active role in inflammation and cell death
• By specifically regulating these enzymes, may be able to better manage inappropriate inflammatory responses, such as those commonly seen in vascular and neurodegenerative disorders |
|
|
feedback inhibition
|
A method of metabolic control in which the end product of a metabolic pathway acts as an inhibitor of an enzyme within that pathway
|
|
|
ribozymes
|
An RNA molecule that catalyzes a biochemical reaction
|
|
|
product
|
The result(s) of a chemical reaction
|
|
|
isozyme
|
• An enzyme that catalyzes the same reaction as another enzyme but at a different optimum temperature
• Because humans and other mammals maintain a fairly stable internal temperature, they have many fewer isozymes than organisms with fluctuating temperatures |
|
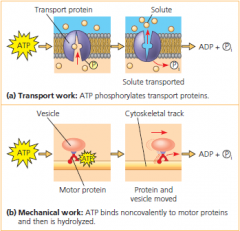
how ATP drives transport and mechanical work
|
• ATP hydrolysis causes changes in the shapes and binding affinities of proteins
• This can occur either (a) directly, by phosphorylation, as shown for a membrane protein carrying out active transport of a solute, or (b) indirectly, via noncovalent binding of ATP and its hydrolytic products, as is the case for motor proteins that move vesicles (and other organelles) along cytoskeletal “tracks” in the cell |

