![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
11 Cards in this Set
- Front
- Back
|
a. Define Metabolism b. State the 2 general purposes of Metabolism |
a. - The sum total of the general chemical processes that occur in living organisms - resulting in growth, production of energy, elimination of waste products etc b. - To capture energy from the environment and convert it to a suitable for cellular work - To transform groups of organic molecules into one another, particularly the transformation of small organic molecules into macromolecules |
|
|
The 2 aspects of metabolism are interdependent - Cells obtain energy by the __(a)__ breakdown of __(b)__ - On the other hand, a major use of stored energy is __(c)__ |
a. exergonic b. organic molecules c. the synthesis of macromolecules |
|
|
a. What are the 2 major routes in which cells can use to obtain energy from the environment? b. Classify the types of organisms which use these 2 different groups + explain how they do this |
a. - Light energy - Energy from large high energy compounds b. Phototrophs (green plants+algae) = light energy - capture light energy via photosynthesis Autotrophs (animal cells) = organic compounds - breaking down of organic compounds which are high in chemical energy (Sugars+Lipids) into smaller products with less energy |
|
|
To ensure a metabolic pathway functions properly, what 2 things must it involve? + explain |
1.) IRREVERSIBLE - Must be highly favoured thermodynamically - So you only get the products 2.) SPECIFIC - The reactions must only give one particular product from the reactants - This allows regulation by enzymes |
|
|
a. Define (in an equation) what the equilibrium constant (Kc) is b. What kind of Kc leads to an irreversible reaction? c. What is the Kc range in which appreciable concentrations of both products and reactants are present at equilibrium? |
a. [products]/[reactants] = Kc b. Very large Kc value c. Kc = 10^-3 - 10^3 |
|
|
a. What are the 2 types of processes which give a spontaneous reaction? b. State the 1st law of thermodynamics c. State the 2nd law of thermodynamics |
a. - Reactions that give out energy (exothermic) - Reactions that increase in disorder (+ve entropy change) b. Total energy is constant. Can be converted but not made or destroyed. c. In any cyclic process, the ENTROPY will increase (or remain the same) |
|
|
a. State the equation for Gibbs free energy changes b. Explain the equation |
a. ΔG = ΔH -TΔS b. - ΔG = Negative for a spontaneous reaction - Negative ΔH (energy/enthalpy) favours spontaneous change - Positive Entropy change favours spontaneous change |
|
|
a. Between Catabolic and Anabolic pathways, which are more likely to be spontaneous + why? b. What does nature use in order to help make various reaction spontaneous? |
a. - Catabolic - You are breaking down molecules, therefore increasing Entropy b. - Enzyme catalysts - Couples unfavourable reactions with highly favourable reactions (e.g. ATP Hydrolysis) |
|
|
a. How can a reaction with a very small Kc (+ve ΔG) become irreversible? b. In the 1st step of Glycolysis, glucose is phosphorylated. This is an irreversible step even though it has a -ve ΔG, how is this achieved? |
a. - By coupling the reaction - With a reaction which has a very negativeΔG and a very large Kc b. By coupling the Phosphorylation reaction with ATP Hydrolysis |
|
|
a. What is Creatine Phosphate used for? b. Where is it found? |
a. - Used as a resevoir of high-potential phosphoryl groups which can be transfer to ADP - i.e. ATP Regeneration b. In vertebrate muscles |
|
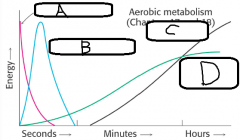
(sources of ATP during exercise) |
a. ATP b. Creatine Phosphate c. Aerobic Metabolism d. Anaerobic Metabolism |

