![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
24 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
aa Structural elements
4ct |
An amino group (-NH2 or -NH3+)
A carboxylic group (-COOH or -COO-) A side chain (R) A hydrogen (H) |
|
|
|
An amino group
|
( -NH2 or -NH3+ )
|
|
|
|
A carboxylic group
|
(-COOH or -COO-{neg})
|
|
|
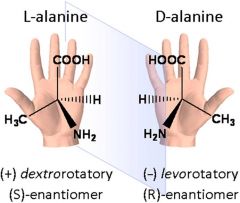
A rule of thumb for determining the D/L isomeric form of an amino acid is the "CORN" rule.
|
The groups: COOH, R, NH2 and H (where R is a side chain). Starting with the hydrogen atom away from the viewer, if these groups are arranged clockwise around the carbon atom, then it is the D-form. If counter-clockwise, it is the L-form.
|
|
|
|
Alanine (Ala, A)
Chemical features = |
Alanine plays a key role in the glucose-alanine cycle: in muscle, alanine and α-ketoglutarate are formed during glucose metabolism. They are recycled to the liver where they feed into gluconeogenesis to reform glucose, which returns to muscle.
|
|
|
|
Built on alanine
2 |
Serine (Ser, S)
Cysteine (Cys, C) |
|
|
|
Based on structure
aliphatic 5 |
ala
gly Leu LLe val |
Having carbon atoms linked in open chains
a g l l v |
|
|
Based on structure
aromatic 3 |
phe
acidic basic |
of or relating to or containing one or more benzene rings
p a b |
|
|
Based on structure
acidic 2 |
asp
glu |
|
|
|
Based on structure
basic 3 |
arg
his lys |
a
h l |
|
|
Based on structure
neutral 4 |
asn
gln ser thr |
a
g s t |
|
|
Based on structure
sulfur 2 |
cys
met |
|
|
|
Based on structure
L-mino acid |
pro
|
|
|
|
Classification of amino acids
polar 11 ct |
arg
asn- asp Cys- gln- glu his lys Ser- thr- Tyr Nm Polar Neutrals from this list 5ct |
Asn
Cys Gln Ser thr |
|
|
Classification of amino acids
nonpolar 10 |
ala
cys gly LLE leu met phe pro trp val |
a
c g l l m p p t v |
|
|
Based on necessity
Essential 9ct |
Histidine
Isolucine Leucine Lysine methionine Phenylalinine threonine Tryptophan valine |
h
i l l m p t t v |
|
|
Based on necessity
Non-essential 11ct |
Alanine
Arginine Aspargine Aspartic Acid Cysteine Glutamic Acid Glutamine Glycine Proline Serine Tyrosine |
a
a a a c g g g p s t |
|
|
Non-essential
|
human body can synthesize them from other compounds
|
|
|
|
Essential
|
human body cannot synthesize them from other compounds at the level needed for normal growth, so they must be obtained from food
|
|
|
|
aromatic
|
relating to or containing one or more benzene rings
|
|
|
|
aliphatic
|
Having carbon atoms linked in open chains
"Hydrocarbons which do not contain a benzene ring are called aliphatic hydrocarbons" |
|
|
|
basic
|
basic side chains at neutral pH. These are =
3ct |
arginine (Arg),
lysine (Lys), histidine (His). |
|
|
21st aa =
3-Letter = 1-Letter = |
Selenocysteine
Sec U |
Selenocysteine has both a lower pKa (5.47) and a higher reduction potential than cysteine. These properties make it very suitable in proteins that are involved in anti-oxidant activity.[4]
Unlike other amino acids present in biological proteins, selenocysteine is not coded for directly in the genetic code.[5] Instead, it is encoded in a special way by a UGA codon, which is normally a stop codon. Such a mechanism is called translational recoding[6] and its efficiency depends on the selenoprotein being synthesized and on translation initiation factors. |
|
|
22nd aa =
3-Letter = 1-Letter = |
Pyrrolysine
Pyl O |
a naturally occurring, genetically coded amino acid used by some methanogenic archaea and one known bacterium in enzymes that are part of their methane-producing metabolism. It is similar to lysine, but with an added pyrroline ring linked to the end of the lysine side chain. It forms part of an unusual genetic code in these organisms, and is considered the 22nd proteinogenic amino acid.
|

