![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
453 Cards in this Set
- Front
- Back
|
Pathological state in which the heart cannot pump blood efficiently enough to meet the metabolic needs of the body.
|
Congestive Heart Failure
|
|
|
What are the four governing factors of systolic function?
|
Contractility, Preload, Afterload, Heart Rate
|
|
|
What leads to decreased myocardial contractility?
|
Loss of functional muscle (MI) or Diffuse deposits in the myocardium (amyloidosis, sarcoidosis, hemochromatosis)
|
|
|
Increased preload (ie. in valve regurgitation) leads to?
|
CHF
|
|
|
This is end diastolic volume and resultant fiber length of the ventricle prior to onset of contraction?
|
Preload
|
|
|
Increased afterload (ie. in aortic stenosis or in severe HTN) leads to?
|
CHF
|
|
|
This is the forsce against which a ventricle that contracts that is contributed to by the vascular resistance especially of the arteries and by the physical characteristics (mass and viscosity) of the blood.
|
Afterload
|
|
|
This is stroke volume X heart rate?
|
Cardiac Output
|
|
|
To maintain arterial pressure and perfusion of vital organs the CV system responds to excessive hemodynamic burden or disturbance in myocardial contractility with the following three mechanisms.
|
Frank starling mechanism
Myocardial hypertrophy Activation of neurohumeral systems |
|
|
This is increased preload of dilation that helps to sustain cardiac performance by enhacing contractility.
|
Frank Starling mechanism
|
|
|
This happens to augment mass of contractile tissue in the heart?
|
Myocardial hypertrophy
|
|
|
This involves the following three things:
1. Release of norepi by adrenergic cardiac nerves leading to increased heart rate and increased myocardial activity 2. Renin angiotensin aldosterone system 3. Release of atrial naturitic peptide |
Activation of neurohumeral systems as an initial compensatory mechanism in CHF
|
|
|
This is the ability of the heart to change its force of contraction and therefore stroke volume in response to changes invenous return.
|
Frank Starling Law
|
|
|
This occurs when the normally functioning heart cannot keep up with the dramatically increased demand for blood flow to one or more organs in the body e.g. in severe anemia, hyperthyroidism, AV fistula, Beriberi, Paget's disease.
|
High output heart failure
|
|
|
True or false, high output heart failure is not a common cause of CHF?
|
True
|
|
|
This occurs in ischemic heart disease, HTN, dilated cardiomyopathy, valvular and paricardial disease.
|
Low output heart failure
|
|
|
This is a B1 or thiamine deficiency, most cases are found in asia due to diets of white rice, or in alcoholic patients, patients who also undergo gastric bypass can suffer from this. This is associated with high output heart failure.
|
Beriberi
|
|
|
This condition happens when the right side of the heart loses its ability to pump blood efficiently.
|
Right sided heart failure
|
|
|
This condition occurs when the left side of the heart cannot pump enough blood to the body.
|
Left sided heart failure
|
|
|
What is the most common cause of RHF?
|
LHF
|
|
|
The principle abnormality is the inability to contract normally and expel sufficient blood.
|
Systolic failure
|
|
|
The principle abnormality is the inability to relax and fill normally (the heart).
|
Diastolic failure
|
|
|
How many classes are in the NY heart association classification?
|
Four
|
|
|
This is when patients have no limitations of activities, they suffer no symptoms from ordinary activities.
|
Class I NYHA classification
|
|
|
This is when patients have slight, mild limitation of activity, they are comfortable with rest or with mild exertion.
|
Class II NYHA classification
|
|
|
This is when patients have marked limitation of activty, they are comfortable only at rest.
|
Class III NYHA classification
|
|
|
This is when patients who should be at complete rest, confined to bed or chair, any physical activity brings on discomfort and Sx occur at rest.
|
Class IV NYHA classification
|
|
|
This is insufficiently sensitive to be useful in predicting outcomes or assessing results of Tx - needs to be based on individual patient. Helps in comparing groups of pts ie. in research studies.
|
NYHA classification
|
|
|
This tracks disease development and progression in heart patients?
|
ACC/AHA staging system
|
|
|
This is when a patient is at high risk for HF but without structural heart dz or Sx of HF; risk factors are HTN, DM, obesity and metabolic syndrome
|
Stage A ACC/AHA staging system
|
|
|
This is when a patient has a structural heart dz but without Sx and Sx of HF, possible Hx of MI, LVH, asymptomatic valvular heart dz, or low EF.
|
Stage B ACC/AHA staging system
|
|
|
This is when a patient has structural heart dz and prior or current Sx of HF.
|
Stage C ACC/AHA staging system
|
|
|
This is when a patient with refractory HF requries specialized intervention.
|
Stage D ACC/AHA staging system
|
|
|
These are all causes of what?
Arrhythmias, MI, PE, Sytemic HTN, Thyrotoxicosis, Pregnancy, Infection, Anemia, Rheumatic and other forms of myocarditis, Physical, dietary, fluid, environmental and emotional excesses, infective endocarditis. |
CHF
|
|
|
What are the causes of primarily systolic failure CHF?
|
CAD, HTN, Dilated cardiomyopathy - idiopathic, toxic (EtOH, doxorubicin), infection (viral parasitic and other)
|
|
|
What are the causes of primarily diastolic CHF?
|
HTN, Hypertrophic cardiomyopathy, Restrictic cardiomyopathy - amyloidosis, sarcoidosis, hemtochromatosis, Constrictive pericarditis, High output failure - chronic anemia, AV shunts, thyrotoxicosis
|
|
|
What are four arrhythmias that show up with CHF?
|
Tachyarrhythmias, A-V dissociation, Abnormal intraventricular conduction, Severe bradycardia
|
|
|
This occurs in CHF, decreased filling time and as a result there is decreased cardiac output. Since increased heart rate increases myocardial oxygen demand, cardiac ischemia may be induced which may lead to decreased contractility.
|
Tachyarrhythmias
|
|
|
This occurs in CHF, results in loss of the atrial contribution to ventricular filling, therefore end diastolic volume is decreased with an associciated decrease in cardiac output.
|
A-V dissociation
|
|
|
This may cause a decrease in synchronicity of contraction with a decrease in myocardial performance. Optimal output requires coordinated impulse propegation and contraction.
|
Abnormal intraventricular conduction
|
|
|
This occurs in the absence oif increased stroke volume can seriously decrease cardiac output and thus precipitate CHF, increased stroke volume may not be possible if the patient has significant heart disease.
|
Severe bradycardia in the absence of increased stroke volume
|
|
|
These two things decrease LV function, and may precipitate CHF.
|
MI and tissue death.
|
|
|
Pt with this has an increased arterial pressure which may worse or cause LV failure.
|
Pulmonary emoblism
|
|
|
Rapid increases in arterial BP w/associated increases in peripheral resistance can increase afterload to an extent sufficient to produce heart failure.
|
Systemic HTN
|
|
|
Up to how many CHF diagnoses made in primary care setting may be wrong?
|
50%
|
|
|
What do you do for the lab workup in CHF?
|
CBC (for e/o infection or anemia), increased BUN and creatinine, decreased Na, increased hepatic enzymes, BNP, TSH, cardiac enzymes, electrolytes K, Mg, and Ca
|
|
|
This helpts distinguish CHF from other causes of acute dyspnea, helps monitor severity but can't distinguish between systolic and diastolic CHF, normal value is under 200.
|
BNP - Brain Natriuretic Peptide
|
|
|
These are not diagnostic for CHF?
|
EKG
|
|
|
EKG's do increase suspicion of CHF when what four things are seen?
|
Previous MI, LV hypertrophy, Left bundle branch block, Tachyarrhythmia e.g rapid afib
|
|
|
This evaluates the chamber dimensions of the heart, valve function, shunts, LVEF, Wall motion abnormalities (suggestive of CAD leading to catherterization/coronary angiography, or patchy myocarditis, LV hypertrophy, pericardial effusion, intracardiac thrombi, tumors and calcifications within heart valves.
|
Echocardiogram
|
|
|
This evaluates how well the heart is pumping. The amount of blood pumped out of a ventricle during each hear beat divided by the total amount of blood in the left ventricle.
|
Ejection Fraction
|
|
|
What is a normal EF (ejection fraction)?
|
50-70%
|
|
|
If you have an EF over 40% it means what?
|
Mainly diastolic function
|
|
|
If you have an EF under 40% it means what?
|
Mainly systolic function
|
|
|
What do you look for on the CXR in CHF?
|
Cardiomegaly, Engorged pulm. vasculature, Pleural fluid R>L (better in upright or decubitus films), thickened fissure between upper and middle lobe, Kerley-B lines
|
|
|
These are short, straight lines in the periphery of the lung lying approximately perpendicular to the pleural surface, caused by increased fluid in the interlobular septa.
|
Kerley-B lines
|
|
|
What are the goals of Tx in CHF?
|
To correct underlying reversible causes, to relieve symptoms, and to prevent woresening of the condition. Tx remains empiric based on small studies and pathophysiologic dz.
|
|
|
What are four things used to Tx systolic dysfunction?
|
Diuretics, ACE inhibitor, Beta-Blocker, Digoxin
|
|
|
With diuretic therapy for systolic dysfunction, what three diuretics are used?
|
Loop diuretics (Furosemide - Lasix 20mg-80mg PO or IV up to TID)
Thiazides in mild to mod CHF Metolazone (Zaroxolyn) in addition to furosemide |
|
|
What are thiings shat should be done when giving diuretics for systolic heart failure?
|
Monitor BP (hypotension) renal fct (prerenal azotemia), electrolytes (natriuresis, kaliuresis), strict ins/outs, daily weights
|
|
|
These causes dilation of arteriolar resistance vessels and venous capacitance vessels leads to decreased preload and afterload
Proven to decrease mortality in patients with CHF Considered part of the initial Tx in addition to diuretic Start low to prevent hypotension Contraindicated in renal insufficiency (cr>3), hyperkalemia, hypotension, allergy |
ACE Inhibitors for CHF Tx
|
|
|
What do you use in CHF Tx if patient is unable to tolerate ACE because of cough?
|
Angiotensin II receptor blockers
|
|
|
Until the mid 90's considered contraindicated, but it causes substantial increase in EF and decrease in LV size, decreased mortality and hospitalization rate.
|
Beta-Blocker
|
|
|
You should start these low in CHF Tx to prevent bradycardia.
|
Beta-Blocker
|
|
|
This is useful secondary to inotropic and vagotonic effect. It is beneficial in pt w/rapid afib, severe CHF, or EF <30%, can be added to diuretic and ACE inhibitor.
|
Digoxin
|
|
|
Decreased hospitalization rate and decreased worsening HF, but no improvement in mortality.
|
Digoxin
|
|
|
You have to give a decreased dose in elderly patients with renal insufficiency.
|
Digoxin
|
|
|
You have to check serum levels of this drug after 7-14 days of TX.
|
Digoxin
|
|
|
The Sx of this are anorexia, nausea, HA, blurred or yellow vision, confusion leading to tachycardia and ventricular fibrillation.
|
Digoxin toxicity
|
|
|
What predisposes patients to digoxin toxicity?
|
Quinidine, Verapamil, decreased kalemia, decreased magnesemia
|
|
|
What is the Tx for digoxin toxicity?
|
Digoxin Fab (ovine)
|
|
|
Tx for this is determined by underlying cause?
|
Diastolic Dysfunction CHF
|
|
|
If you have HTN as the underlying cause of diastolic dysfunction CHF what do you give for TX?
|
CCB, ACEI, BB, Diuretics (careful since higher filling pressure may be needed to maintain cardiac output)
|
|
|
If you have aortic stenosis as the underlying cause of diastolic dysfunction CHF what do you give for TX?
|
Diuretics, Valve replacement
|
|
|
If you have aortic regurgitation and mitral regurgitation as the underlying cause of diastolic dysfunction CHF what do you give for TX?
|
ACEI to increase cardiac output and decrease pulmonary wedge pressure, together with diuretics.
|
|
|
If you have mitral stenosis with decreased emptying of the LA as the underlying cause of diastolic dysfunction CHF what do you give for TX?
|
Diuretics if Afib is present, give digitalis, verapamil and or BB, valve replacement or balloon valvuloplasty.
|
|
|
What are the lifestyle modifications for helping with the TX of diastolic dysfunction CHF?
|
Weight loss, fluid intake restrictions to less than 1.5L, sodium restrictions (<2g day), regular aerobic exercise tailored to the pt’s tolerance level, abstinence from EtOH, daily body weight (monitor for fluid retention)
|
|
|
What is a sign of fluid retention?
|
If the pts weight goes up five lbs over after a few days it can show signs of holding onto fluid.
|
|
|
In which, diastolic or systolic dysfunction CHF, is morbidity and mortality 50% lower?
|
Lower in diastolic dysfunction
|
|
|
This is a multisystem disease resulting from an autoimmune reaction to infection with group A streptococci.
|
Acute Rheumatic Fever
|
|
|
Almost all manifestations of acute rheumatic fever resolve completely, except for what?
|
Cardiac valvular damage (rheumatic heart disease) which may persist after the other features have disappeared.
|
|
|
Up to what percentage of patients with ARF progress to RHD?
|
Up to 60% of patients with ARF progress to RHD
|
|
|
ARF is mainly a disease of children aged?
|
5-14
|
|
|
The prevalence of RHD peaks between what ages and is more common in what gender?
|
RHD peaks between 25 and 40 years and is more common in females.
|
|
|
ARF is exclusively caused by infection of the upper respiratory tract with any strain of what?
|
Group A strep
|
|
|
There is a latent period of how many weeks between the precipitating group A strep infection and the appearance of the clinical features of ARF?
|
Three weeks
|
|
|
What is the most common clinical presentation of ARF?
|
Polyarthritis and fever
|
|
|
What are four other clinical presentations, besides polyarthritis, of ARF?
|
Carditis, Chorea, Erythema marginatum, Subcutaneous nodules
|
|
|
What is the hallmark of heart involvement with ARF?
|
Valvular damage from endocardial involvement
|
|
|
What valve is almost always affected with ARF?
|
Mitral valve
|
|
|
Myocardial inflammation with ARF may lead to what in the EKG?
|
P-R interval prolongation
|
|
|
Pericardial involvement with ARF may lead to what?
|
Pericarditis
|
|
|
To qualify as a major manifestation this must have inflammation of more than one joint in ARF.
|
Joint involvement
|
|
|
ARF almost always affects what?
|
Large joints and is symmetrical
|
|
|
What do you give for joint involvement in ARF?
|
Highly responsive to salicylates or other NSAIDS
|
|
|
This commonly occurs in the absence of other manifestions in ARF, it follows a prolonged latent period after infection, particularly affects the head (causing characteristic darting movements of the tongue) and the upper limbs. May be generalized or restricted to one side of the body. Varies in severity from very mild to interfering ADLS, eventually resolves completely within six weeks.
|
Sydenham’s chorea
|
|
|
This is the classic rash of ARF, begins as pink macules that clear centrally, leaving a serpiginous, spreading edge, evanescent appearing and disappearing before the examiner’s eyes. Occurs usually on the trunk, sometimes on the limbs, but almost never on the face.
|
Erythema marginatum
|
|
|
This is a painless, small (0.5-2cm), mobile lump that is beneath the skin near tendons or overlying bony prominences, particularly of the hands, feet, elbows, occiput, and occasionally the vertebrae. This is associated with ARF.
|
Subcutaneous nodules
|
|
|
Apart from erythema marginatum, and subcutaneous nodules, what are other features of ARF?
|
Fever occurs in most cases of ARF, Elevated acute phase reactants (CRP and ESR) are also present in most cases, mild leukocytosis is occasionally seen.
|
|
|
What is essential in making the diagnoses of ARF
|
Evidence of a preceding group A streptococcal infection
|
|
|
Most cases of ARF do not have a what?
|
Most cases off ARF do not have a positive throat culture or rapid antigen test, so serologic evidence is usually needed.
|
|
|
What are the most common serologic tests associated with ARF?
|
Anti-streptolysin O (ASO) and anti-DNase B (ADB) titers
|
|
|
When you are making a diagnosis of ARF what do you need to do?
|
There is no definitive test, Jones criteria refers to clinical features, major and minor criteria, To make the diagnosis group A streptococci PLUS 2 major criteria or 1 major and 2 minor criteria.
|
|
|
What are the major criteria for diagnosing ARF?
|
Carditis, Migratory polyarthritis, subcutaneous nodules, erythema marginatum, chorea
|
|
|
What are the minor criteria for diagnosing ARF?
|
Arthralgia, fever, elevated acute phase reactants, prolonged PR interval
|
|
|
What are the three exceptions to the Jones criteria?
|
Chorea is only manifestation, indolent carditis as only manifestation, and recurrent rheumatic fever.
|
|
|
If ARF is untreated it last on average how long?
|
12 weeks
|
|
|
With treatment, patients with ARF are usually discharged from hospital within?
|
1-2 weeks
|
|
|
Echocardiography should be obtained in patients with what in ARF?
|
Patients with cardiac findings
|
|
|
With the exception of Tx of heart failure, which may be life saving, the Tx of ARF is what?
|
Symptomatic
|
|
|
To treat the precipitating group a strep infection what do you use?
|
Penicillin is the drug of choice, erythromycin may be used if Pen allergy
|
|
|
For Tx of arthritis, arthralgia, and fever, associated with ARF, what do you give?
|
Salicylates and NSAIDS, Aspirin is the drug of choice
|
|
|
Many clinicians treat cases of severe carditis, associated with ARF, with what?
|
Glucocorticoids – Prednisone or prednisolone are recommended. IV methylprednisolone may be used in very severe carditis
|
|
|
Medications do not alter the outcome of this. Milder cases can usually be managed by providing a calm environment.
|
Chorea
|
|
|
In patients with severe chorea, associated with ARF, what do you give?
|
Carbamazepine or sodium valproate are preferred, or IV immunoglobulin
|
|
|
In the follow up to ARF Tx what should be done?
|
Inflammatory markers should be monitored every 1-2 weeks until they have normalized, echocardiogram after 1 month, if carditis is present pt should be informed of need for antibiotic prophylaxis against endocarditis for dental and surgical procedures.
|
|
|
What should be done with patients who had ARF after they are TX
|
Patients should be entered onto the local ARF registry and contact made with a PCP to ensure a plan for follow up and administration of secondary prophylaxis before the patient is discharged.
|
|
|
What is the primary prevention of ARF?
|
Tx of group A streptococcal pharyngitis
|
|
|
What is the secondary prevention of ARF?
|
Long term penicillin prophylaxis to prevent recurrences, Benzathine penicillin G delivered every four weeks or more frequently, also oral penicillin V can be given instead but less effective, if Pen allergy erythromycin can be given.
|
|
|
What are two other things that are post-streptococcal syndromes that may be confused with rheumatic fever?
|
Post streptococcal reactive arthritis
Pediatric Automimmune neuropsychiatric disorders associated with streptococcal infection. |
|
|
How do you differentiate Post Streptococcal Reactive Arthritis from RF?
|
Small joint involvement that is often symmetric, A short latent period following strep infection, occasional causation by non group A hemolytic strep infection, slower responsiveness to salicylates, the absence of other features of ARF, PARTICULARLY CARDITIS.
|
|
|
This is a term that links a range of tic disorders and obsessive compulsive symptoms with group A streptococcal infections.
|
PANDAS
|
|
|
This is infection or inflammation of the heart valves or the lining of the heart.
|
Endocarditis
|
|
|
This is endocarditis caused by any microorganism
|
Infective endocarditis
|
|
|
This most commonly involves heart valves (native or prosthetic). It may occur on the mural endocardium where it is damaged by aberrant jets of blood or foreign bodies. May occur on intracardiac devices themselves.
|
Infective endocarditis
|
|
|
This is a hectically febrile illness that rapidly damages cardiac structures, hematogenously seeds extracardiac sites, and, if untreated, progresses to death within weeks.
|
Acute endocarditis
|
|
|
What three pathogens are primarily responsible for acute endocarditis?
|
Hemolytic streptococci, S. Aureus, Pneumococci
|
|
|
This follows an indolent course, causes structural cardiac damage slowly, if at all, rarely metastasizes, gradually progressive unless complicated by a major embolic event or ruptured mycotic aneurysm.
|
Subacute endocarditis
|
|
|
What typically causes subacute endocarditis?
|
Viridans streptococci, enterococci, HACEK group, Bartonella species.
|
|
|
What are the predisposing factors for endocarditis?
|
Congenital heart disease, chronic rheumatic heart disease, degenerative valve disease, prosthetic valves, intracardiac devices, IV drug use, health care associated infection.
|
|
|
This is from IV catheter infections, nosocomial wound and urinary tract infections, chronic invasive procedures such as hemodialysis, transvenous pacemaker lead – and/or implanted defibrillator associated endocarditis.
|
Health Care-Associated Native Valve Endocarditis
|
|
|
This can come from the oral cavity, skin and upper respiratory tract are the respective primary portals for viridans streptococci, staphylococci, and HACEK organisms, streptococcus bovis from the GI tract, enterococci enter the bloodstream from the genitourinary tract.
|
Community acquired native valve endocarditis
|
|
|
What organisms are a part of the HACEK organisms?
|
Haemophilus, Actinobacillus, Cardiobacterium, Eikenella, and Kingella
|
|
|
This is generally the result of an intraoperative or postoperative complication, coagulase negative staphylococcus, S. Aureus, facultative gram negative bacilli, diphtheroids, and fungi.
|
Prosthetic valve endocarditis arising within 2 months of valve surgery.
|
|
|
Pathogens are similar to those in community acquired native valve endocarditis.
|
Prosthetic valve endocarditis arising over 12 months after surgery.
|
|
|
This is commonly caused by E. aureus, many of which are MRSA, when involving the tricuspid valve. Left sided infections have a more varied etiology and involve abnormal valves, often involving Pseudomonas aeruginosa and Candida species, with sporadic cases involving bascillus, lactobacillus, and corynebacterium species.
|
IV Drug Use associated with endocarditis
|
|
|
This is more common among injection drug users than among patients who do not inject drugs.
|
Polymicrobial endocarditis
|
|
|
This accounts for 5 to 15% of endocarditis cases, may be due to prior antibiotic exposure, may be due to fastidious organisms.
|
Culture negative endocarditis
|
|
|
Endothelial injury causes aberrant flow and allows either direct infection by virulent organisms or the development of an uninfected platelet fibrin thrombus – a condition called what?
|
Nonbacterial thrombotic endocarditis (NBTE)
|
|
|
The nonbacterial thrombotic endocarditis subsequently serves as a site of what?
|
Serves as a site of bacterial attachment during transient bacteremia
|
|
|
Fibrin deposition combines with platelet aggregation to generate an infected what?
|
Vegetation
|
|
|
In the absence of host defenses, organisms enmeshed in the growing platelet fibrin vegetation proliferate to form what?
|
Dense microcolonies
|
|
|
The pathophysiologic consequences and clinical manifestations of endocarditis arise from?
|
Damage to intracardiac structures, embolization of vegetation fragments, leading to infection or infarction of remote tissues, hematogenous infection of sites during bacteremia, tissue injurly due to the deposition of circulating immune complexes or immune responses to deposited bacterial antigens, constitutional symptoms probably result from cytokines.
|
|
|
What are the clinical manifestations of endocarditis?
|
Fever, chills, sweats, anorexia, weight loss, malaise, myalgias, arthralgias, back pain, heart murmur, aterial emboli, spenomegaly, clubbing, neurologic manifestations, peripheral manifestations, Petechiae
|
|
|
What are the peripheral manifestations of endocarditis?
|
Osler’s nodes, subungal hemorrhages, janeway lesions, Roth’s spots
|
|
|
What are the lab manifestations with endocarditis?
|
Anemia, leukocytosis, microscopic hematuria, elevated erythrocyte sedimentation rate, elevated C-reactive protein level, rheumatoid factor, circulating immune complexes, decreased serum complement
|
|
|
In patients with subacute endocarditis, fever is typically what?
|
Low grade and rarely exceeds 103 F
|
|
|
In patients with acute endocarditis, fever is typically what?
|
Temperatures of 103-104 F are often noted
|
|
|
Fever may be blunted or absent in patients who are what?
|
Elderly or severely debilitated or who have marked cardiac or renal failure.
|
|
|
What are the cardiac manifestations of endocarditis?
|
Heart murmurs, heart failure, heart block, abscess, MI
|
|
|
If you see heart murmurs with endocarditis it means?
|
Usually indicative of the predisposing cardiac pathology, valvular damage and ruptured chordae may result in new regurgitant murmurs. If involving a normal valve, heard on presentation in only 30-45% of patients but ultimately detected in 85%.
|
|
|
If you see heart failure with endocarditis it means?
|
Develops in 30-40% of patients, usually a consequence of valvular dysfunction but occasionally is due to endocarditis associated myocarditis or an intracardiac fistula.
|
|
|
This is usually from extension of infection into paravalvular tissue adjacent to the aortic valve. Occasionally from abscess near mitral valve.
|
Heart block in endocarditis.
|
|
|
This happens from extension of infection beyond valve leaflets into adjacent annular or myocardial tissue, which in turn may cause fistulae with new murmurs. May extend into pericardium, causing pericarditis.
|
Abscess in endocarditis
|
|
|
This can occur from emboli to coronary artery.
|
MI in endocarditis.
|
|
|
What are five non cardiac manifestations in endocarditis?
|
Embolic events, septic embolization, neurologic symptoms, musculoskeletal symptoms, renal complications, splenic abscess, mycotic aneurysms.
|
|
|
These are often seen with infarction, may involve extremities, spleen, kidneys, bowel or brain, associated with non cardiac manifestations of endocarditis.
|
Embolic events
|
|
|
These are common with S. Aureus, vegetation over 10mm in diameter and those located on the mitral valve are the most likely to embolize, tricuspid involvement may cause septic pulmonary emboli, associated with non cardiac manifestation of endocarditis.
|
Septic embolization
|
|
|
These are most often resulting from embolic strokes, other complications include aseptic or purulent meningitis, microabscesses in brain and meninges, intracranial hemorrhage due to hemorrhagic infarcts or ruptured mycotic aneurysms, seizures, and encephalopathy, associated with non cardiac manifestation of endocarditis.
|
Neurologic symptoms
|
|
|
Inflammatory arthritis and back pain, must be distinguished from local metastatic infection, associated with non cardiac manifestation of endocarditis.
|
Musculoskeletal symptom
|
|
|
Embolic renal infarcts cause flank pain and hematuria, glomerulonephritis, from immune complex deposition on the glomerular basement membrane, associated with non cardiac manifestation of endocarditis.
|
Renal complications
|
|
|
Half of these cases involve the cerebral arteries, extracerebral aneurysms present as local pain, a mass, local ischemia or bleeding, associated with non cardiac manifestation of endocarditis.
|
Mycotic aneurysms
|
|
|
What are the peripheral manifestations associated with endocarditis?
|
Petechiae, Osler’s Nodes, Janeway Lesions, Roth Spots
|
|
|
These are peripheral manifestations associated with endocarditis that show up on the palate, conjunctiva, or beneath the fingernails. They can also show up as splinter hemorrhages – Linear reddish brown lesions of the nailbed.
|
Petechiae
|
|
|
These are tender violaceous raised lesions on the finger pads, toes or feet that are associated with peripheral manifestation in endocarditis.
|
Osler’s Nodes
|
|
|
These are macular erythematous, blanching, non painful lesions on the palms or soles of the feet, associated with peripheral manifestation in endocarditis.
|
Janeway Lesions
|
|
|
These are exudative lesions in the retina, associated with peripheral manifestation in endocarditis.
|
Roth Spots
|
|
|
What are the specific predisposing conditions for endocarditis? 4 of them.
|
Early onset prosthetic valve endocarditis, late onset prosthetic valve endocarditis, IV drug use, health care associated endocarditis.
|
|
|
This lacks peripheral vascular manifestations, and typical symptoms, may be obscured by comorbidity associated with recent surgery. It is a specific predisposing condition for endocarditis.
|
Early-onset prosthetic valve endocarditis
|
|
|
This presents with typical clinical features, and It is a specific predisposing condition for endocarditis.
|
Late-onset prosthetic valve endocarditis
|
|
|
This is seen in almost 50% of endocarditis, infection is limited to the tricuspid valve, these patients present with fever, faint or no murmur, and prominent pulmonary findings. It is a specific predisposing condition for endocarditis.
|
IV Drug Use
|
|
|
This arises after recent hospitalization, or it is a direct consequence of long term indwelling devices. It is a specific predisposing condition for endocarditis.
|
Health care associated endocarditis
|
|
|
This is established with certainty only when vegetations obtained at cardiac surgery, at autopsy, or from an artery (an embolus) are examined histologically and microbiologically.
|
Diagnosis of infective endocarditis.
|
|
|
This has highly sensitive and specific diagnostic schema for endocarditis.
|
Duke Criteria
|
|
|
If you have two major criteria, one major and three minor criteria, or five minor criteria allows a clinical diagnosis of what?
|
Definite endocarditis
|
|
|
Alternative diagnosis is established, symptoms resolve and do not recur with 4 days of antibiotic therapy, surgery or autopsy after four days of antimicrobial therapy yields no histologic evidence of endocarditis.
|
Rejected endocarditis
|
|
|
Illnesses not classified as definite endocarditis or rejected, one major and one minor criterion or three minor criteria.
|
Possible endocarditis.
|
|
|
Positive blood culture – typical microorganisms for infective endocarditis from two separate blood cultures, persistently positive blood culture, blood cultures drawn 12 hours apart or all of three or a majority of four or more separate blood cultures. Single positive blood culture for Coxiella burnetii or antibody titer of >1:800
|
Major Duke Criteria
|
|
|
Evidence of endocardial involvement – positive echocardiogram, intracardiac mass on valve or supporting structures or in the path of regurgitant jets or in implanted material, or abscess, or new partial dehiscence of prosthetic valve, or new valvular regurgitation.
|
Major Duke Criteria
|
|
|
What are the five minor Duke criteria
|
1.Predisposing heart condition or IV drug use, 2. Fever 38C/100.4 F, 3. Vascular phenomena: major arterial emboli, septic pulmonary infarcts, mycotic aneurysm, intracranial hemorrhage, conjunctival hemorrhages, janeway lesions, 4. immunologic phenomona: glomerulonephritis, Osler’s nodes, Roth’s spots, rheumatoid factor, 5. Microbiologic evidence: Positive blood culture not meeting major criterion or serologic evidence.
|
|
|
In the absence of prior antibiotic therapy, three blood culture sets separated from each other by at least 1 hour, should be obtained from different venipuncture sites over how many hours to help diagnose endocarditis?
I |
24 hours
|
|
|
If cultures remain negative after 48-72 hours, how many additional blood culture sets should be obtained to diagnose endocarditis?
|
Two or three.
|
|
|
This cannot image smaller vegetations, may be technically inadequate in some patients, and is not adequate for evaluating prosthetic valves or detecting intracardiac complications in endocarditis.
|
Transthoracic echocardiography
|
|
|
This is significantly more sensitive than TTE, and is the optimal method for the diagnosis of prosthetic endocarditis or the detection of myocardial abscess, valve perforation, or intracardiac fistulae.
|
Transesophageal echocardiography
|
|
|
f you have a negative transesophageal echocardiography when endocarditis is likely, what does it mean?
|
does not exclude the diagnosis but rather warrants repetition of the study in 7–10 days.
|
|
|
What should not be administered initially to hemodynamically stable patients with subacute endocarditis, especially those who have received antibiotics within the preceding 2 weeks.
|
Empirical antimicrobial therapy
|
|
|
Patients with acute endocarditis or with deteriorating hemodynamics who may require urgent surgery should be treated empirically after?
|
Three sets of blood cultures are obtained.
|
|
|
It is difficult to eradicate bacteria from vegetations because?
|
this site is relatively deficient in host defenses and because non-growing, metabolically inactive bacteria are less easily killed by antibiotics.
|
|
|
To cure endocarditis, all bacteria in the vegetation must be killed; therefore, therapy must be?
|
bactericidal and prolonged. Generally given parenterally.
|
|
|
The minimum inhibitory concentration (MIC) of penicillin for the causative isolate must be determined with this.
|
Streptococcal Endocarditis
|
|
|
Killing requires the synergistic interaction of a cell wall–active antibiotic and an aminoglycoside
Enterococci causing endocarditis must be tested for high-level resistance to streptomycin and gentamicin, -lactamase production, and susceptibility to penicillin and ampicillin |
Enterococcal Endocarditis
|
|
|
Regimen is based on the presence or absence of a prosthetic valve or foreign device, the native valve(s) involved, and the resistance of the isolate to penicillin and methicillin
|
Staphylococcal Endocarditis
|
|
|
What patients with endocarditis are able to complete threapy as outpatients?
|
If the patient is fully compliant and have sterile blood cultures, are afebrile during therapy, and have no clinical or echocardiographic findings that suggest an impending complication they may complete therapy outpatients.
|
|
|
What three things should be done when monitoring antimicrobial therapy in endocarditis?
|
Serum concentrations of aminoglycosides and vancomycin
Blood tests to detect renal, hepatic, and hematologic toxicity Blood cultures should be repeated daily until sterile, rechecked if there is recurrent fever and performed again 4-6 weeks after therapy to document cure. |
|
|
People who have endocarditis and also have congesitive heart failure, perivalvular infection, uncontrolled infection, S. aureus endocarditis or patients that need preventntion of systemic emboli may need?
|
Intracardiac surgery
|
|
|
What factors adversely affect the outcome of endocarditis?
|
Older age, severe comorbid conditions, delayed diagnosis, involvement of prosthetic valves or the aortic valve, an invasive or antibiotic resistant pathogen, intracardiac complications, major neurologic complications.
|
|
|
This has been recommended by the American Heart Association in conjunction with selected procedures considered to entail a risk for bacgeremia and endocarditis.
|
Antibiotic prophylaxis
|
|
|
What procedures require prophylaxis when a patient has had endocarditis?
|
All dental procedures that involve manipulation of the gingival tissue or the periapical region of the teeth or perforation of the oral mucosa, respiratory tract procedures that involve incision of the respiratory mucosa, Procedures on infected skin, skin structure, or musculoskeletal tissue.
|
|
|
Prophylaxis for patients who have had endocarditis is not required for what?
|
Gastrointestinal tract procedures, genitourinary tract procedures, routine dental anesthetic injections through noninfected tissue, dental radiographs, placement of removable prosthodontic or orthodontic appliances, adjustment of orthodontic appliances, placement of orthodontic brackets, shedding of deciduous teeth, bleeding from trauma to the lips or oral mucosa.
|
|
|
What are the high risk cardiac lesions for which endocarditis prophylaxis is advised before dental procedures?
|
Prosthetic heart valves, prior endocarditis, unrepaired cyanotic congenital heart disease, for six months following complete repair of a congenital heart defect, incompletely repaired congenital heart disease with residual defects adjacent to prosthetic material, valvulopathy devloping after cardiac transplantation.
|
|
|
What is the standard oral regimen for prophylaxis of endocarditis in adults with high risk cardiac lesions?
|
Amoxicillin 2 grams 1 hour before procedure
|
|
|
What is the Rx for pts unable to take oral medications, but need prophylaxis of endocarditis in adults with high risk cardiac lesions?
|
Ampicillin 2 grams IV or IM wihtin one hour of procedure
|
|
|
What is the ABX regimen for prophylaxix of endocarditis in adults with high risk lesions if someone has a penicillin allergy?
|
Clarithromycin or azithromycin, cephaxalin, clindamycin
|
|
|
What is the ABX refimen for prophylaxis of endocarditis in adults with high risk cardiac lesions if the patient has a penicillin allergy and is unable to take PO meds?
|
Cefazolin or ceftriaxone, or clindamycin
|
|
|
Heart “grows” from outside of pericardial cavity into cavity
Three layers result what are they? |
Fibrous pericardium and Serous pericardium
|
|
|
This is the outermost layer, fibrous, rigid, and adhered to surround surfaces?
|
Fibrous pericardium
|
|
|
This is made up of two layers of pericardium.
|
Serous pericardium
A. Parietal pericardium – closely attached to the fibrous pericardium B. Visceral pericardium – adherent to the outer surface of the myocardium |
|
|
The intent of this is to allow free movement of the heart throughout the cardiac cycle. It also limits distention of the cardiac chambers and works to increase the heart’s efficiency.
|
the pericardial space and the fluid that fills it
|
|
|
This is thin, clear, straw colored fluid, normally void of cells
|
Pericardial fluid
|
|
|
This is inflammation of the pericardium.
|
Pericarditis
|
|
|
This is when there is an increase in pericardial fluid.
|
Pericardial fluid
|
|
|
This is an increase in pericardial fluid that interferes with proper filling of the ventricles.
|
Cardiac tamponade
|
|
|
This is surgical perforation of the pericardium for the purpose of draining the pericardial space and obtaining pericardial fluid for analysis.
|
Pericardiocentesis
|
|
|
What is the most common form of pericarditis observed?
|
Acute pericarditis
|
|
|
This has less than six weeks since symptom onset, it is classified as infectious, non-infectious, idiopathic, and as post cardiac injury.
|
Acute pericarditis
|
|
|
This is when there is 6 weeks to 6 months of symptoms and is known as effusive-constrictive pericarditis.
|
Subacute pericarditis
|
|
|
This is when there has been symptoms over six months in pericarditis.
|
Chronic constrictive pericarditis
|
|
|
What are the top three causes of acute pericarditis?
|
Neoplastic, Autoimmune and Viral - adenovirus, enterovirus, cytomegalovirus, influenza virus, hepatitis B virus and herpes simplex virus.
|
|
|
A patient with the following symptoms shows the cardinal manifestations of what?Chest pain, pericardial friction rub, characteristic ECG changes, Pericardial effusion +/- cardiac tamponade, Paradoxical pulse
|
Acute pericarditis
|
|
|
Chest pain that is steady, severe, constricting, retrosternal, radiating to the neck, arms and left shoulder, OFTEN RELIEF WITH SITTING UP, LEANING FORWARD, BUT WORSE WITH LYING SUPINE, can be pleuritic - aggrivated by inspiration, cough, change in body position.
|
Acute pericarditis chest pain
|
|
|
Chest pain in acute pericarditis can look like what two things?
|
AMI or STEMI
|
|
|
This is audible in up to 85% of patients, one, two, or three components, high pitched, rasping, scratching, or grating sounds, heard best along Lower Left Sternal Border with diaphragm, Accentuate with patient sitting upright, leaning forward, end expiration.
|
Pericardial Friction Rub
|
|
|
What is seen in stage one of acute pericarditis ECG changes?
|
Stage 1: widespread ST elevation with ST depressions in aVr and or V1
|
|
|
What is seen in stage two of acute pericarditis ECG changes?
|
Days later return of ST segments to baseline
|
|
|
What is seen in stage three of acute pericarditis ECG changes?
|
Days later T wave inversion
|
|
|
What is seen in stage four of acute pericarditis ECG changes?
|
Weeks later return to baseline
|
|
|
What is the Tx ofr acute pericarditis?
|
Rule out MI, Anti-inflammatories - Aspirin, Ibuprofen, Selective COX-2 inhibitors, Colchicine. Steroids, ABX - if bacterial cause, Discontinue anticoagulant therapy - may lead to hemopericardium
|
|
|
On x-ray, usually obvious widening of cardiac silhouette. But what else can cause this finding? à enlarged heart (from CHF for example) So how do you tell the difference between say CHF and Acute pericarditis?
|
On physical exam of two patients, both with enlarged silhouette on chest xray, the one with reduced or muffled heart sounds will likely have pericardial effusion.
the one with normal or louder than normal heart sounds will have cardiomegaly |
|
|
This is when there is accumulation of fluid in the pericardial space, an inflammatory response or bleeding, causes enlargement of cardiac silhouette on chest X-Ray (Water Bottle configuration) and Ewart's sign
|
Acute pericarditis: Pericardial Effusion
|
|
|
This is when the base of the left lung is compressed by pericardial fluid causing dullness, increased fremitus, and egophony beneath angle of left scapula. Associated with acute pericarditis: pericardial effusion.
|
Ewart's sign
|
|
|
How do you diagnose an effusion in acute pericarditis?
|
Transthoracic echo is gold standard
|
|
|
This is an echo-free space between posterior pericardium and left ventricular epicardium.
|
Small effusion <100mL
|
|
|
This is an echo-free space between anterior right ventricle and anterior pericardium.
|
Large effusion >500mL
|
|
|
This is the accumulation of fluid in the pericardial space in a quantity sufficient to cause serious obstruction to the inflow or blood to the ventricles.
|
Acute pericarditis: Cardiac Tamponade
|
|
|
To help distinguish from heart failure, remember the hallmark findings of tamponade that aren’t found with heart failure, including:
|
-Reduced QRS amplitude
-Electrical alternans |
|
|
In general, a rapid accumulation of a small amount of fluid (<200 mL) can produce?
|
Cardiac Tamponade
|
|
|
A slow progressive accumulate of a large amount of fluid (>2000 mL) may not produce tamponade because?
|
The pericardium has had time to distend appropriately to accommodate the excess fluid and pressure.
|
|
|
What can slow onset cardiac tamponade in pericardial effusion resemble?
|
Dyspnea, orthopnea, hepatic engorgement = Heart Failure
|
|
|
What are the diagnostic criteria for acute pericarditis: cardiac tamponade?
|
Beck's Triad - Hypotension, Soft or absent heart sounds, Jugular venous distension
Paradoxical Pulse |
|
|
What is a greater than normal (10 mmHg) inspiratory decline in systolic arterial pressure?
|
Paradoxical pulse
|
|
|
What do you do immediately to confirm the suspicion of tamponade?
|
Echocardiogram
|
|
|
What is the Tx ofr cardiac tamponade in acute pericarditis?
|
Pericardiocentesis
|
|
|
This is the surgical removal of pericardial fluid.
|
Pericardiocentesis
|
|
|
How do you perform a pericardiocentesis?
|
Echo guided insertion of needle into pericardial space - subxiphoid approach, 5th or 6th left intercostal space, insertion of catheter for drainage, fluid drained and collected, analyzed for cells, and cultures obtained, drain left in place until drainage ceases.
|
|
|
This occurs after resolution of acute pericarditis, particularly in patients with tuberculosis origin, but many other causes such as post radiation/cancers breast, lung, lymphoma, Trauma, Cardiac Surgery, Autoimmune Dz, Chronic renal failure with uremia.
|
Chronic Constrictive Pericarditis
|
|
|
Everything in chronic constrictive pericarditis results in what?
|
Results in deposition of granulation tissue in pericardium
|
|
|
Impaired diastolic function due to restricted filling, poor forward flow: weakness and fatigue, Fluid retention: Weight gain, increased abdominal girth, abdominal discomfort and edema, Kussmaul's sign, and congestive hepatomegaly are all seen with what?
|
Chronic Constrictive Pericarditis
|
|
|
What is an elevation of the neck veins during inspiration?
|
Kussmaul's sign
|
|
|
This is impaired hepatic function and possibly jaundice, ascites (looks like cirrhosis)
|
Congestive hepatomegaly
|
|
|
What is seen on the ECG, CXR, Echo and CT or MRI with chronic constrictive pericarditis?
|
ECG: Low voltage of QRS, diffuse T wave flattening or inversion
CXR: Usually normal silhouette Echo: Pericardial thickening CT or MRI: Pericardial thickening and calcifications |
|
|
What is the TX for chronic constrictive pericarditis?
|
Pericardial resection
|
|
|
Pericardial resection entails?
|
Removing about as much as possible of the pericardium. It is the only definitive treatment of CCP Operative mortality is 5-10% and increases with severity, so early treatment is key.
|
|
|
What are the most common aortic aneurysms?
|
Infrarenal aortic aneurysms are most common.
|
|
|
What is the male to female ration of those who have infrarenal abdominal aortic aneurysms?
|
4:1 male:female
|
|
|
What are significant comorbidities for aortic aneurysm?
|
HTN, COPD, CAD
|
|
|
An abdominal aortic aneurysm is ?
|
Over 50% increase in normal aortic diameter
|
|
|
AAA has associated vascular diseases such as?
|
CAD, HTN, DM, Hyperlipidemia, Tobacco, COPD, CVD +/- stroke, claudication, visceral and renovascular occlusive disease
|
|
|
What is an independent risk factor for mortality in AAA?
|
Chronic renal insufficiency
|
|
|
90-95% of proximal neck anatomy is what in AAA?
|
Infrarenal
|
|
|
What is the primary etiology with AAA?
|
Degenerative 80%, Dissection 15%
|
|
|
What age should a first degree relative of someone with AAA be screened?
|
45-50
|
|
|
What are the pathogenic - genetic causes of AAA?
|
Enzyme deficiencies, Connective tissue disorders
|
|
|
This is when AAA is due to lysl oxidase deficiency
|
collagen and elastin cross linking.
Enzyme deficiencies - pathogenesis of AAA genetic |
|
|
What are the two particular connective tissue disorders associated with genetic pathogenesis of AAA?
|
Marfan Syndrome - abnormal fibrillin, Structural component of elastin, Ehlers-Danlos (type IV) - Abnormal type III collagen
|
|
|
When you have predilection for formation in distal aorta, Gradual tapering - less elastin, more collagen, - devoid of vasa vasorum, Reflected pressure waves from the peripheral arterial tree increases mural tension in the infrarenal aorta.
|
Pathogenesis of AAA - Mechanical
|
|
|
Degenerative aneurysms often coexist with?
|
Generalized atherosclerosis, therefore are referred to as: atherosclerotic aneurysms.
|
|
|
Focal intimal thickening encroaches on lumen leading to?
|
Consequent compensatory arterial dilation
|
|
|
In AAA there is a loss of normal arterial architecture due to what?
|
Media thins underneath the plaque in atherosclerosis
|
|
|
Aortic structural integrity and stability are dependent upon what two things?
|
Media: Musculoelastic fascicles and Adventitia: Collagen
|
|
|
When you have degredation of the media and adventitia this leads to what?
|
Aneurysmal dilation - decrease quantity of elastin, increased activity of elastase, increased collagenase activity, decrease in the concentration of protease inhibitors.
|
|
|
What diagnostic methods are used to find AAA?
|
Physical exam, X-Ray, Aortography, US, CT Scan, MRI/MRA
|
|
|
In the physical exam, the aorta must be?
|
5cm to be palpated, prominent aortic pulsation experienced
|
|
|
In X-ray with AAA you will see?
|
A fine rim of calcification
|
|
|
With ultrasound in AAA you will see?
|
Initial evaluation of pulsatile mass or F/U accurate to 3mm
|
|
|
What is an excellent technique to image the abdominal aorta and its branches?
|
CT angiogram / Spiral CT
|
|
|
What are the indications for an aortography in AAA?
|
Renovascular HTN, unexplained impaired renal function, symptoms of visceral angina, iliofemoral occlusive disease, horseshoe or pelvic kidney, prior colectomy.
|
|
|
When determining operative risk for repairing AAA, what are the independent risk factors for mortality?
|
– CRI (creat >1.8)
– CHF – CAD (ischemia on EKG) – COPD – Elderly |
|
|
When should AAA be treated?
|
When long term risk of AAA related death exceeds risk of repair plus risk of death following repair.
|
|
|
How is risk of AAA related death predicted?
|
AAA size (diameter) has been the only reliable indicator of rupture and death.
|
|
|
What are the four instances that AAA is a surgical emergency?
|
When they are symptomatic patients showing Rupture - 50% immediate mortality, over 75% overall mortality, Aortoenteric fistula, Aortocaval fistula, Vague abdominal pain - constant or throbbing, epigastric radiating to the back
|
|
|
What constitutes a rapid progressive enlargement of an AAA?
|
Enlarging over .5 cm/yr
|
|
|
What are the reasons why treat an aortic aneurysm?
|
Thrombosis, embolization - blue toe syndrome, Fistulization - enteric, caval, Local compression, Atypical/Morphologic - False, mycotic, penetrating ulcer, saccular, inflammatory, Symptomatic - Pain, rupture.
|
|
|
What is involved with open repair of an AAA?
|
Direct exposure - transabdominal retroperitoneal, Proximal and distal control, ligation of branches, Prosthetic graft sutured to normal artery.
|
|
|
This is when a device is inserted from remote site, passed intraluminally under radiologic guidance, secured by expandable stent attachment system, used to fix AAA.
|
Endovascular AAA repair
|
|
|
What are the anatomic criteria for endovascular AAA repair candidacy?
|
Infrarenal neck, Aortic bifurcation, Iliac arteries and access vessels
|
|
|
This is an intimal tear allowing blood to separate the intimal and medial layers of the aorta.
|
Aortic dissection, acute is within 2 weeks of onset, chronic is over 2 weeks.
|
|
|
What is no an associated risk factor for aortic dissection?
|
Atherosclerosis
|
|
|
50% of dissections in women under 40 occur during?
|
Pregnancy
|
|
|
What is the primary associated risk factors for aortic dissection?
|
Hypertension and Connective Tissue Disorders
|
|
|
What is the clinical presentation of aortic dissection?
|
Chest Pain – “Worst pain ever”
Death Stroke Cardiac Tamponade Lower extremity ischemia Syncope Renal Failure Mesenteric Ischemia Spinal Cord Ischemia Voice Hoarseness |
|
|
What is seen in the CXR with aortic dissection?
|
– Widening of mediastinum
– Low sensitivity, low specificity |
|
|
What is seen with the aortography with aortic dissection?
|
– False lumen visualized 87%*
– Intimal flap in 70%* – Intimal tear/entry site 56%* |
|
|
What imaging study has excellent sensitivity/specificity for aortic dissection?
|
MRI
|
|
|
What is seen with CT in aortic dissection?
|
– Excellent sensitivity/specificity
– Excellent detail resolution – Limitation: sensitivity slightly decreased at ascending aorta – False lumen larger than true lumen 90% of time* |
|
|
What is the TX for type A aortic dissection?
|
– Ascending arch replacement +/- valve repair/replacement
– Mortality increases 1% per hour* – 60% in-hospital mortality with medical management** |
|
|
What is the TX for type B aortic dissection?
|
– Uncomplicated: no end organ ischemia, meaning no malperfusion
• medical therapy with emphasis on decreasing blood pressure – Intravenous B-blocker – Intravenous arterial dilator – Complicated: end organ ischemia, meaning malperfusion • Surgery with graft replacement • Endovascular Therapy |
|
|
One of the most challenging vascular problems Time is of the essence Patients often in generally poor health Questions remain as to optimal therapy - surgical vs. percutaneous Many will require a combination of modalities Multi-disciplinary involvement important Successful outcomes dependent upon experienced team
|
Acute limb ischemia
|
|
|
Acute limb Ischemia comes from embolism, what are the cardiac sources?
|
• Atrial Fibrillation
• Myocardial infarction • Prosthetic valves • Atrial myxomas • Endocarditis • Rheumatic heart disease • In-situ thrombosis • Bypass graft occlusion • Aortic dissection |
|
|
Acute limb Ischemia comes from embolism, what are the non cardiac sources?
|
• Atherosclerotic disease of proximal vessels
• Mural thrombus of aortoiliac, femoral, popliteal aneursyms • Tumor emboli • Paradoxical embolus |
|
|
When acute limb ischemia is assocaited with Low flow state what are the two etiological factors?
|
– Cardiogenic shock
– Systemic sepsis |
|
|
When acute limb ischemia is assocaited with Drugs what are the two types of drugs often seen?
|
– Cocaine
– Vasopressors |
|
|
When acute limb ischemia is associated with Vasculitides
|
– Takyasu’s arteritis
|
|
|
What involving the knee can be a part of acute limb ischemia?
|
Popliteal entrapment
|
|
|
What Hypercoagulable states can be a part of acute limb ischemia?
|
– Protein C/S deficiency, malignancy
|
|
|
When Trauma is involved in acute limb ischemia what is seen?
|
– Supracondylar humerus fracture
– Posterior knee dislocation – High velocity penetrating injury – Iatrogenic (endovascular, a-lines) |
|
|
Factor - Embolism Fill in the rest. . .
Often Detected - Source - Claudication - Physical Findings - |
Embolism - Factor
Often detected – Afib Atherosclerotic lesion - Source Often not present - Claudication Normal contralateral pulses - Physical Findings - Minimal atherosclerosis, sharp cutoff, few collaterals Angiogram |
|
|
Factor - Thrombosis - Fill in the blanks. . .
Often detected Source Claudication Physical Findings |
Thrombosis - Factor
No discrete - Source Frequently present - Claudication Contralateral evidence of PVD Physical Findings - Diffuse atherosclerotic disease, tapered and irregular cutoff, collaterals Angiogram |
|
|
This is associated with a history of claudication - may be absent in 40% of patients.
|
Acute arterial thrombosis
|
|
|
This usually arises with no history of claudication.
|
Acute arterial embolism - emboli usually occur in the setting of atrial fib, 80% AF, 10% prior MI, 10% other aneurysms
|
|
|
Why does it matter to know the difference between embolism and thrombus?
|
Can affect decision on first line therapy, pts subjected to balloon embolectomy in setting of acute arterial thrombosis have a particularly poor outcome.
|
|
|
What are the six P's assocaited with acute limb ischemia?
|
• Pain
• Paresthesia • Paralysis • Pallor • Pulselessness • Poikilothermia |
|
|
This is usually the first symptom with acute limb ischemia.
|
Pain
|
|
|
This is a sign of progressive ischemia, loss of proprioception and light sensation early.
|
Paresthesia
|
|
|
Reflects ischemic myopathy, intrinsic muscles of the foot, flexors/extensors of lower leg, indicative of need ofr emergent revascularization.
|
Paralysis
|
|
|
White, waxy leg in absence of collateral circ.
– In arterial thrombosis, this may gradually improve due to collaterals • May have mottling – Fixed cyanosis • Capillary thrombosis and rupture • Terminal ischemia |
Pallor
|
|
|
This is an absolute prerequisite for acute limb ischemia diagnosis.
|
Pulselessness
|
|
|
Intact pulses in contralateral leg suggests?
|
Embolic etiology
|
|
|
Absent pulses in contralateral leg supports?
|
Diffuse PAD and thrombosis
|
|
|
What are exceptions to pulselessness?
|
Venous gangrene and blue toe syndrome
|
|
|
This is coolness compared to contralateral limb, both cool in aortic occlusion.
|
Poikilothermia
|
|
|
If you have Acute painful, cold leg, but no neuro deficit, and audible pedal arterial doppler signals what does this mean?
What is involved with category IIa and IIb of the rutherford scale? |
• Category I. Viable, on the rutherford scale. Often acute thrombosis of chronic atherosclerotic artery or previous bypass graft
• Time for angiographic assessment • Institution of lytic therapy may be possible and beneficial • Anticoagulation while awaiting urgent angiography • Operative balloon thrombo-embolectomy alone unlikely to successfully treat arterial thrombosis or stenosed artery or graft |
|
|
What is involved with category IIa and IIb of the rutherford scale?
|
– IIA: minimal sensory loss, no muscle weakness
• Time for angiography with CLOSE SURVEILLANCE of limb – IIB: Greater sensory and motor dysfunction • Immediate revascularization indicated Further Class II information • Acute pallor, no prior claudication history, palpable contralateral pulses, AFIB • Embolism diagnosis most likely • But in many cases, differentiating etiology challenging • Debate: Does angiography waste valuable time, or provide beneficial information?? |
|
|
What class of the rutherford scale follows this criteria?
– Cold cyanotic limb, calf muscle rigidity – Patients often systemically very ill • Severe comorbidities, nonambulatory – Primary amputation may be appropriate |
Class III Rutherford Irreversible
|
|
|
Initial bolus followed by continuous infusion
– Decrease thrombus propagation OPTIONS Angiography Thrombolytic therapy, endovascular revascularization Percutaneous Mechanical thrombectomy Open surgical intervention |
Heparin Therapy and options associated
|
|
|
What are the guides to TX in acute limb ischemia?
|
Site and extent of occlusion
– Embolus versus thrombus – Native artery versus bypass graft – Duration of ischemia – Patient co-morbidities – Contraindications to thrombolysis or surgery |
|
|
What are the advantages of the cath lab in ALI?
|
– Angiographic capabilities
• Roadmap anatomy – Therapeutic possibilities • Thrombolytic infusion • Percutaneous mechanical thrombectomy • Subsequent endovascular treatment of exposed underlying lesion |
|
|
What are the disadvantages of the cath lab in ALI?
|
– “Time-factor”: clinical situation may not allow for time required for restoration of adequate arterial flow with lysis
– Endovascular therapy may not allow complete revascularization – Need for continuing surveillance of limb, with need to re-image at any time if clinical deterioration |
|
|
Establish inflow
Balloon embolectomy catheters passed up iliac If inadequate inflow, intraop agram may identify chronic lesion treatable by additional endovascular or surgical means – Establish outflow Balloon catheters for PFA, SFA, tibial embolectomy Over the wire balloons under fluoro aid in thrombus retrieval – Completion angiogram |
Embolectomy via CFA exploration
|
|
|
Use of open surgical procedures in ALI has been shown to have high rates of?
|
Peri-op morbidity and mortality
– Rates of amputation and death up to 25% each |
|
|
Thrombolysis is attractive in Tx ALI because?
|
– Restoring patency, unmasking culprit lesion, allowing non-surgical Tx in some cases
– Latest devices/adjunct for thrombolysis may enhance, accelerate lytic results |
|
|
What are the criticisms for using thrombolysis in ALI TX?
|
• Timing: slow rate of thrombus dissolution in some clinical situations
• Clinical ischemic time may not allow for lysis • May require repeated imaging to assess progress • Potential for distal embolism during lysis • Risk of re-thrombosis • Hemorrhagic risks |
|
|
What are the absolute contraindications for using thrombolysis to TX ALI?
|
• Absolute
– CVA within past 3 months – Active bleeding diathesis – Recent GI bleeding – Neurosurgery or head trauma within past 3 months |
|
|
What are the relative contraindications to using thrombolysis to TX ALI?
|
– Major non-vascular surgery or trauma within 10 days
– Uncontrolled HTN – Intracranial tumor – Recent eye surgery – Puncture of non-compressible vessel |
|
|
What are the minor contraindications for using thrombolysis to TX ALI?
|
– Hepatic failure with coagulopathy
– Bacterial endocarditis – Pregnancy – Diabetic hemorrhagic retinopathy |
|
|
Often insufficient as stand-alone therapy
• grafts have a 2 year patency rate of 79% if underlying lesion is uncovered v. 9.8% if not • Even if a subsequent bypass is needed, it may allow surgical bypass in more elective setting |
Thrombolysis in TX of ALI
|
|
|
Signs/symptoms
– Weakness on toe extension and foot dorsiflexion – Pain on passive toe flexion, foot plantar flexion – Hypesthesia in dorsal first web space – Anterior compartment tense |
Anterior Compartment Syndrome, ALI post revascularization
|
|
|
Signs/symptoms
– Weakness of toe flexion and foot inversion – Pain on passive toe extension, foot eversion – Hypesthesia plantar aspect foot and toes – Tense deep post. Compartment • Between tibia and Achilles tendon |
Deep posterior compartment syndrome ALI Post revascularization
|
|
|
If you have compartment syndrome, what can be done to alleviate it?
|
4 compartment fasciotamy
|
|
|
• One of the most complex decision pathways in vascular disease
• Treatment must be based upon severity of ischemia, rather than cause • Time permitting, an arteriogram can add helpful diagnostic and anatomic information, and can be therapeutic – should be considered in all appropriate patients • Team approach can lead to improved outcomes – Restoration of perfusion can require both catheter-directed and surgical techniques |
Acute Limb Ischemia Summary
|
|
|
• Systemic panarteritis of the medium & large arteries
• Chronic inflammation • Also known as Temporal Arteritis, temporal artery is most commonly involved • Etiology is unknown • Frequently associated with Polymyalgia Rheumatica, ½ of pts with Giant cell arteritis also have PMR • Over the age of 50, peak age is 60-80 years old • Twice as common in women & more common in whites than African Americans |
Giant Cell Arteritis
|
|
|
What are the signs of Giant Cell Arteritis?
|
• Localized HA or scalp pain
• Temporal artery tenderness or decreased temporal artery pulse • Visual Sx (amaurosis fugax or visual loss) • Jaw claudication—pain in the jaw with movement such as eating or talking, throat pain, fever |
|
|
Any patient over age 50 with fever of unknown origin, normal WBC & very high ESR…..must r/o?
|
Giant Cell Arteritis even if no HA or other signs & symptoms
|
|
|
The most important reason to Dx & Tx giant cell arteritis is to prevent?
|
Blindness
|
|
|
What Is the hallmark of possible blindness with giant cell arteritis?
|
The hallmark is elevation of acute phase reactants, which is an acute inflammatory response, such as ESR & CRP. ESR is typically over 50, but a lower ESR doesn’t r/o the Dx
|
|
|
What do you do to diagnose giant cell arteritis?
|
Unilateral temporal artery bx (side that is Sx, tender or inflamed) within 1-2 weeks of initiating prednisone.
|
|
|
What length must you get In the temporal biopsy to adequately diagnose giant cell arteritis?
|
3-5 cm In length because the disease Is segmental
|
|
|
A positive biopsy of giant cell arteritis shows what?
|
Inflammatory changes with an Infiltration of lymphocytes, histiocytes, plasma cells and giant cells.
|
|
|
The results of a temporal artery bx remain positive for as long as __________ after Initiating treatment with prednisone. Never delay tx a patient with prednisone because of fear of the bx not showing Inflammation because of starting steroids.
|
4 weeks
|
|
|
When a pt has Sx suggestive of temporal arteritis, they are immediately started on?
|
• prednisone 60 mg QD before a bx is even obtained, Continue prednisone 60 mg QD for 1-2 months then start tapering the dose when the symptoms have resolved.
• Start taking one aspirin a day |
|
|
What is the role of ESR when TX giant cell arteritis?
|
• Use the ESR to adjust the steroid dose.
• As the ESR decreases, the disease activity is diminishing and you can decrease the steroid dose. • Discontinue the prednisone when the ESR reaches normal range |
|
|
What is the prognosis of giant cell arteritis?
|
• Blindness rarely occurs when the ESR is in the normal range.
• With prompt treatment, the prognosis is very good & Sx start to resolve over several days to months. • Visual Sx are usually permanent. • The average duration of treatment is usually 2 years. • Morbidity from steroid treatment is often worse than the underlying disease. |
|
|
What is a presentation of Arterial peripheral vascular disease?
|
Atherosclerosis
|
|
|
What are three Venous presentations of peripheral vascular disease?
|
Varicose Veins
Thrombophlebitis Chronic Venous Insufficiency |
|
|
What race is more at risk of facing peripheral vascular disease?
|
African American
|
|
|
A disorder caused by atherosclerosis that limits blood flow to the limbs.
|
Peripheral Arterial Disease (PAD)
|
|
|
A symptom of PAD characterized by pain, aching, or fatigue in working skeletal muscles. It arises when there is insufficient blood flow to meet the metabolic demands in leg muscles of ambulating patients.
|
Intermittent Claudication (IC)
|
|
|
What are the systemic manifestations of atherosclerosis?
|
• TIA or Ischemic Stroke
• MI or Unstable Angina • Renovascular Hypertension • Intestinal Ischemia • Erectile Dysfunction • Intermittent Claudication • Critical Limb Ischemia, rest pain, gangrene, ulcers, amputation |
|
|
What are some of the features associated with the underlying causes of PAD?
|
• Characterized by lipid deposits in intima of large and medium-sized arteries
• Associated with fibrosis/calcification • Invariably a systemic condition • Clinical recognition varies in individuals |
|
|
The hallmark of atherosclerosis is?
|
atherosclerotic plaque
|
|
|
Plaque is most commonly at?
|
bifurcations or bends
– Local stress flow – Turbulent blood flow – Stasis of blood |
|
|
What are the modifiable risk factors of atherosclerosis?
|
• Smoking
• Hypertension • Diabetes • Hyperlipidemia • Obesity • Hyperhomocysteinemia |
|
|
What are the nonmodifiable risk factors of atherosclerosis?
|
Age, Sex, Genetics
|
|
|
What do the following represent as a risk factor for atherosclerosis?
• Accelerates atherosclerosis 200%–400% • Risk of coronary artery ischemic events increases 2–4 times • Results in 4 times risk of stroke • PAD develops a decade earlier than nondiabetics • CV risk equivalent to 3 non-diabetic risk factors |
Diaebetes Mellitus
|
|
|
The single most important risk factor for developing PAD
• PAD progresses faster and cuts longevity of revascularization procedures • Increases the rate of limb amputation • Significantly decreases patient survival |
Smoking
|
|
|
This has a synergistic effect, dramatically raising risk when combined with any other risk factor.
|
Smoking
|
|
|
This is associated with
– Metabolic abnormalities stemming from reduced blood flow and O2 delivery – Significant reduction (50%) in muscle fibers compared with controls – Smaller type I and II muscle fibers with greater arterial ischemia – Hyperplastic mitochondria and demyelination of nerve fibers |
Intermittent claudication
|
|
|
Intermittent claudication is 2 to 4 times more common among persons with?
|
Diabetes
|
|
|
What Is the ADA/AHA Standardized approach to PAD?
|
• Annual history for exercise-induced leg pain in all diabetic patients
• Annual palpation of leg pulses for all adult patients with diabetes • ABI should be performed for insulin-dependent diabetic patients >35 years of age, or patients with ³20 years’ duration of diabetes |
|
|
How do you diagnose and assess the severity of peripheral vascular disease?
|
Vascular history, physical examination, ankle brachial index measurement, noninvasive vascular laboratory tests.
|
|
|
What are the symptoms to describe a functional description of Intermittent claudication?
|
• Symptoms
– Exertional aching pain, cramping, tightness, fatigue – Occur in muscle groups, not joints (buttocks, hips, legs, calves) – Are reproducible from one day to the next on similar terrain – Resolves completely with 2-5 minutes of rest |
|
|
When you are doing the arterial physical exam for PAD in the lower extremities you do what?
|
• Auscultate abdomen for bruits
• Palpate for abdominal aortic aneurysm • Palpate femoral, popliteal, posterior tibial, and dorsalis pedis pulses • Inspect feet for ulcers, fissures, calluses – Evaluate overall foot skin care |
|
|
Type of ulcer located on feet, toes and heel, pale foot , dependent rubor, yellow, brown or black color, ulcer appears punched out.
|
Arterial (ischemic)
|
|
|
Type of ulcer located at pressure points on bottom of feet, callous surrounding a punched out ulcer, color varies depending on circulation
|
Neuropathic (diabetic)
|
|
|
Type of ulcer below the knee and medially, base of ulcer is red, lots of drainage, irregular borders, venous stasis skin discoloration, shiny smooth skin, leg edema
|
Venous ulcer
|
|
|
What are the non-invasive vascular tests?
|
• ABI measurements
• Pulse-volume recordings • Segmental pressure measurements • Duplex ultrasonography • Treadmill exercise Noninvasive Vascular Tests |
|
|
Ankle sytolic pressure/Brachial artery systolic pressure =
|
ABI
|
|
|
Both ankle and brachial systolic pressures should be taken using a what?
|
Hand held doppler
|
|
|
For ABI is 95% sensitive and 99% specific for?
|
PAD
|
|
|
ABI
0.90–1.30 0.70–0.89 0.40–0.69 less than or equal to 0.40 >1.30 |
Interpretation
Normal Mild Moderate Severe Noncompressible vessels |
|
|
What are ofur indications for referral ofr vascular specialty care?
|
Lifestyle-disabling claudication (refractory to exercise or pharmacotherapy), Rest pain, Ischemic ulcers, gangrene
|
|
|
What are the two medications used to Tx intermittent claudication?
|
Pentoxifylline and Cilostazol
|
|
|
What is a new medication for the Tx of PAD that reduces ischemic events, but doesn't improve claudication symptoms?
|
Clopidogrel (Plavix)
|
|
|
What new medication for patients with PAD doesn’t reduce ischemic events, but it does improve claudication symptoms?
|
Cilostazol
|
|
|
Patients with intermittent claudication have systemic atherosclerosis and are at increased risk for?
|
MI and stroke
|
|
|
What are the major lipid risk factors in PAD?
|
– Elevated LDL-C
– Elevated triglyceride level – Low HDL-C |
|
|
What are the four indications for revascularization for intermittent claudication?
|
• Lifestyle-limiting symptoms
• Continued disability despite appropriate nonsurgical management • Technically feasible revascularization options exist • Expectation of favorable risk/benefit ratio |
|
|
What are the goals with limb revascularization?
|
• Relieve limb-threatening ischemia
• Treat claudication (after other measures) |
|
|
What are the indications for surgical intervention in PAD/PVD?
|
• Gangrene
• Non-healing ulcers • Ischemic rest pain • Claudication causing lifestyle deterioration refractory to pharmacologic intervention and behavioral modification |
|
|
Ache, pain, numbness of arch of foot/toes with leg elevation, Most uncomfortable at night while resting in bed, Interferes with sleep, Relief with dependent positioning of limb
|
Ischemic rest pain
|
|
|
Found distally at ends of toes, over bony prominences on feet
• Dry, devitalized, black • Intense pain |
Ischemic ulceration
|
|
|
Endovascular techniques for the TX of PAD includee what four things?
|
PTA
Atherectomy Stents Thrombolytic therapy |
|
|
Provides an anatomic assessment or a map for revascularization
• Not indicated in the routine diagnostic evaluation of patients with symptomatic PAD, except if claudication is disabling. • absolutely indicated in patients who have critical limb ischemia, a condition characterized by ischemic pain at rest or tissue loss. • If the patient is being considered for a revascularization procedure. |
Arteriography
|
|
|
These are all manifestations of atherosclerois. Because of the generalized nature of atherosclerosis, the three disorders frequently occur together, but they may not be symptomatic.
|
PAD, CAD, CVD
|
|
|
• Smoking cessation
• Lipid control – LDL-C, £ 100 mg/dL – Raise HDL-C – Lower triglycerides • BP control – Use ACE inhibitors • Diabetes control – HbA1C £ 7.0% • Antiplatelet therapy – ASA, clopidogrel • Achieving ideal body weight • Exercise |
Factors that may improve atherosclerosis
|
|
|
When reevaluating a patient in follow up care for PAD 90 days after initation of therapeutic program what should you do?
|
– Assess symptomatic status of limb
– Reassess atherosclerotic risk factor intervention and antiplatelet therapy – Review compliance with home exercise therapy – Consider pharmacologic therapy for nonresponders – Continue monitoring every 90 days until patient improves – Thereafter, monitor every 6 months |
|
|
• Involves the deep veins of the leg or arm.
• Can cause life threatening emboli to the lungs, venous dysfunction, and chronic swelling. |
Deep Venous Thrombosis
|
|
|
This describes 3 factors that are critically important in the development of venous thrombosis:
|
Rudolf Virchow (Virchow’s Triad)
|
|
|
What makes up Rudolf Virchow (Virchow’s Triad)?
|
• (1) venous stasis
• (2) activation of blood coagulation • (3) vein damage. |
|
|
• The end result is early thrombus interaction with the endothelium.
• Stimulates local cytokine production • Leukocytes adhere to the endothelium • Over time, thrombus development begins with the infiltration of inflammatory cells into the clot. |
Results of Virchow's Triad
|
|
|
What is one of the highest risk factors for venous thrombosis?
|
Antithrombin deficiency
|
|
|
What are the risk factors for DVT?
|
• Prior history of DVT
• Age 30-80 • Venous stasis/immobilized patients • Malignancy due to abnormal coagulation • Post surgery • Patients taking HRT…estrogen or birth control pills • Pregnant women • Smoking |
|
|
What are the symptoms of DVT?
|
• Are due to obstruction of venous drainage
• Pain • Tenderness • Unilateral leg swelling • Warmth • Erythema • Palpable cord • Pain with dorsiflexion of foot (Homan’s Sign) |
|
|
True or False, DVT can not be diagnosed or excluded based on clinical findings.
|
True
|
|
|
Describe the correlation between DVT and Cellulitis?
|
• Severe venous congestion produces a clinical appearance that can be indistinguishable from that of cellulitis.
• Patients with a warm, swollen, tender leg should be evaluated for both cellulitis and DVT because patients with DVT often develop a secondary cellulitis. • Patients with primary cellulitis often develop a secondary DVT. • Superficial thrombophlebitis is often associated with a clinically inapparent DVT. |
|
|
What is the study of choice to diagnose DVT?
|
Ultrasound
|
|
|
In the lab analysis for DVT this test shows degradation products of fibrin and plasmin. This is a sign of positive clot formation.
|
D-Dimer
|
|
|
What are the hypercoagulable studies performed in the lab analysis of DVT?
|
• Hypercoagulable Studies
– Antithrombinn deficiency – Protein C deficiency – Protein S deficiency – Factor V Leiden – Hyperhomocysteinemia – Anticardiolipin Antibodies |
|
|
How do you prevent DVT?
|
• Compression
• Anticoagulation – Subcutaneous heparin – Early prophylaxis is associated with a significant reduction in post op thrombosis – Greatest effect if initiated within 8 hours of surgery – Continue for 7-10 days after surgery |
|
|
What is the medical TX of DVT?
|
Anticoagulation, Thrombolysis, Vena Caval Filters
|
|
|
What is the mainstay of medical therapy for DVT?
|
Anticoagulation
• The mainstay of medical therapy. – Continuous IV heparin until systemic anticoagulation is achieved (in patient) – Low molecular weight heparin as QD or bid subcutaneous injection (out patient) • Rapid anticoagulation in first 24 hours of Dx to prevent recurrent venous thrombosis during first 3 months from 25% to 5%. |
|
|
This is used for Long term anticoagulation
• Vitamin K antagonist • Interrupts the production of Vitamin K–dependent coagulation factor production by the liver. • The effect is delayed by 72 hours until the existing circulating coagulation factors are cleared. • During this period, heparin anticoagulation is important to prevent worsening thrombosis. • INR maintained between 2.0 to 3.0 |
Warfarin
|
|
|
The first episode venous thrombosis or thrombotic event due to a transient reversible risk factor should be treated for at least?
|
Three months
|
|
|
First episode Idiopathic venous thrombosis, Tx length should be?
|
6-12 months
|
|
|
This Is recommended for patients with recurrent episodes of venous thrombosis regardless of the cause.
|
Indefinite therapy
|
|
|
Long-term therapy with LMWH has been shown to be as effective as warfarin in the treatment of venous thrombosis, except in those patients with?
|
A concurrent malignancy
|
|
|
What are the contraindications to warfarin TX?
|
• Hemorrhagic stroke
• Active bleeding • Intracranial Neoplasm • Malignant hypertension • Recent major surgery • Recent significant trauma • Severe thrombocytopenia • Pregnancy |
|
|
This is a TX for DVT that does the following things:
• Catheter based reduction of the thrombus with intrathrombus injection of plasminogen. • Preserves valve function • It accelerates the body’s own natural thrombolytic pathway. |
Thrombolysis
|
|
|
This was developed to trap emboli and minimize venous stasis in DVT.
|
IVC Filter
|
|
|
Deep venous thrombosis, with or without pulmonary embolism, in patients with contraindications for anticoagulation, is the most common indication for an?
|
IVC Filter
|
|
|
What are the indications for an IVC filter?
|
• DVT or PE with contraindication for anticoagulation
• Recurrent thromboembolism while on anticoagulation • Anticoagulation complications requiring termination of therapy • Large, free-floating iliofemoral thrombus in high-risk patients • Enlarging iliofemoral thrombus while on anticoagulation • Chronic PE in patient with pulmonary hypertension and cor pulmonale • Patient with significant fall risk |
|
|
Progression of DVT and PE can occur despite full therapeutic anticoagulation in?
|
13% of patients
|
|
|
This develops as venous thrombi break off from their location of origin and travel through the right heart and into the pulmonary artery, causing a ventilation perfusion defect and cardiac strain.
|
Pulmonary Embolism
|
|
|
What Is the relationship between DVT and CVI?
|
• Venous thrombosis causes an injury to the endothelial layer initiating inflammation that leads to vein wall fibrosis.
• Decreased vein wall contractility and valve dysfunction causes chronic venous insufficiency. • The increase in venous pressure causes symptoms of varicose veins, lower extremity edema, and venous ulceration. |
|
|
normal veins that have dilated under the influence of increased venous pressure
|
Varicose veins and Spider veins
|
|
|
This is the result of venous insufficiency due to valve incompetence in the deep or superficial veins.
|
Elevated venous pressure
|
|
|
These are the undesirable pathways by whichh venous blood refluxes back into the congested extremity.
|
Varicose veins
|
|
|
Prevalance of venous insufficiency
|
– Telangiectasias : 80% in men and 85% in women
– Varicose veins : 40% in men and 16% of women – Ankle edema : 7% in men and 16% in women – Venous ulcers : 1% |
|
|
What are the risk factors for venous insufficiency?
|
– Caucasian
– older age – female – obesity – pregnancy – prolonged standing – taller height |
|
|
What are the symptoms of varicose veins?
|
• leg heaviness
• exercise intolerance • pain or tenderness along the course of a vein • pruritus • burning sensations • restless legs • night cramps • edema • skin changes • paresthesias |
|
|
Pain caused by this often improves with walking or by elevating the legs in contrast to the pain of arterial insufficiency, whichh is worse with ambulation and elevation.
|
Venous insufficiency
|
|
|
Pain and other symptoms associated with venous insufficiency may worsen with what?
|
Menstrual cycle, pregnancy, and in response to exogenous hormonal therapy (oral contraceptives)
|
|
|
How many stages are associated in rating venous insufficiency?
|
0-6, 6 being the worst
|
|
|
What is the goal of using imaging studies with venous insufficiency?
|
To identify and map all areas of acute or chronic obstruction and all areas of reflux within the deep and superficial venous systems.
|
|
|
What is the standard imaging used for venous insufficiency?
|
Color-flow duplex ultrasonography
|
|
|
Imaging study for venous insufficiency:
• Most specific & most sensitive for pelvis and legs where other imaging can’t reach. • Can detect nonvascular causes off leg pain and edema. |
MRI
|
|
|
• Invasive
• Infuses radiographic contrast material into the veins. • Good for difficult cases • Nearly 15% of patients undergoing this for detection of deep venous thrombosis (DVT) develop new thrombosis after contrast associated with this. |
Venography
|
|
|
These must not be removed or sclerosed in regards to venous insufficiency.
|
Hemodynamically helpful varices
|
|
|
Superficial varicosities are the inevitable result of high-pressure flow into a normally low-pressure system.
|
If there's NO deep system obstruction
|
|
|
What type of varicosities are hemodynamically harmful?
|
Varicosities carrying retrograde flow
|
|
|
What are the nonsurgical txs for venous insufficiency?
|
Compression, sclerotherapy, laster therapy
|
|
|
What are the surgical txs for venous insufficiency?
|
Endovenous laser therapy, radiofrequency ablation, phlebectomy
|
|
|
What are the grade levels ofr compression stockings?
|
Grade 1-VI with Six being the most compression
|
|
|
What are the tips and tricks with compression stockings?
|
Put them on in the morning, wear them as part of your work "uniform, take them off at night, replace stockings every 3-6 months
|
|
|
• A sclerosing substance is injected into the vein to produce endothelial destruction which causes a fibrotic cord and eventually reabsorption of all vascular tissue layers.
• Local treatment of the superficial varicosities of venous insufficiency is unsuccessful if the underlying areas of reflux have not been found and treated. |
Sclerotherapy
|
|
|
• Effective for tiny surface spider veins (those found on the face).
• Not useful as primary therapy for treatment of spider veins of the lower extremity. • More painful • High variability of light absorption in the skin |
External Laser Therapy
|
|
|
• Thermal ablation technique that uses a laser fiber inside the vein.
• Under ultrasound guidance, local anesthetic is injected around the vessel separating it from its fascial sheath. |
Endovenous Laser Therapy
|
|
|
• New thermal ablation technique that uses a special catheter placed inside the vein.
• Thermal sensors record the temperature within the vessel. • Energy is delivered until the tissue temperature is sufficient to ensure endothelial ablation. |
Radiofrequency Ablation
|
|
|
• Allows removal of short segments of vein through tiny incisions along the length of the vein.
• A day procedure |
Phlebectomy
|
|
|
• Occurs in the gaiter distribution
• Ferritin and ferric oxide deposition • Treatments – Compression stockings – Evaluate and treat the GSV/LSV |
Pigmentation
|
|
|
• Delayed tissue healing
• Treatments – Compression stockings – Unna boot – Evaluate and treat the GSV/LSV |
Ulceration
|
|
|
• Contents
– Zinc oxide – Calamine – Glycerin • Covered with Ace bandage • Changed weekly • Don’t use on patients with arterial disease |
Unna Boot
|
|
|
What are the chronic venous disease Key Points
|
• Different patient population
• Distinguish venous vs. arterial ulcers • Compression stockings for relief • Appropriate treatment • Compression stockings for prevention Beware of patients with PAD and CVI |
|
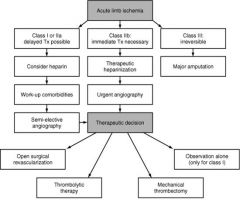
|

|

