![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
121 Cards in this Set
- Front
- Back
|
Why is it necessary to compartmentalize DNA in the nucleus to pretect it?
|
To protect it from the harsh cytosolic environment
|
|
|
Why is it necessary to separte ribosomes from pre-mRNA in the nucleus?
|
The nucleus separates ribosomes from pre-mRNA to prevent early translation before mRNA processing has occurred
|
|
|
What are the components of the nuclear envelope?
|
2 bilayered membranes, perinuclear space, and pores(for mRNA and ribosomes)
|
|
|
What happens in the nucleolus of the nucleus? Is it a separate compartment?
|
rRNA synthesis takes place in the nucleolus, which is NOT a separate compartment
|
|
|
What types of chromatin are constituitive and facultative?
|
Constituitive are dark, dense, inactive heterochromatin that are NEVER transcribed, while facultative chromatin may or may not become euchromatin(unwound, active)
|
|
|
What is another term for the inner and outer nuclear membranes of the nucleus? What is betwen them?
|
Lamina layers are separated by intramembrane/perinuclear space
|
|
|
What is the perinuclear space and the outer membrane continuous with?
|
The ER and the rER
|
|
|
What surround the cytoplasmic side of the nucleus?
|
Intermediate filaments, ribosomes
|
|
|
What supports the cytoplasmic side and the inner membrane of the nucleus?
|
intermediate filaments
|
|
|
What enables nuclear transport? what energy source is used for this?
|
Nuclear pore complexes - requires ATP
|
|
|
How many molecules pass through 3000-5000 pores each minute?
|
3000-5000
|
|
|
What is imported and exported through the nuclear pore complexes?
|
Proteins and snRNPs are imported mRNA and RNPs are exported
|
|
|
what proteins make up the nuclear pore complex?
|
Glycoproteins
|
|
|
What do nuclear pore complexes resemble in microscopy?
|
Baskets
|
|
|
what causes the nuclear pore "baskets" to open/close?
|
calcium
|
|
|
What amino acids comprise
the nuclear localization signals on cargo proteins being imported into the nucleus? |
Arg. and Lys. are on cargo proteins, and act as a nuclear localization signal "zip code" on these proteins that are destined for the nucleus
|
|
|
What is required to translocated cargo proteins into the nucleus throguh the nucleoporins?
|
Importin is the passort to get in the nucleus, but requires ATP
|
|
|
What dissociates the importin complex from the cargo proteins once they have been translocated into the nucleus?
|
Nuclear Ran-GTP
|
|
|
What are the small G proteins? What are their functions?
|
Ras, Rho, Rab, Ran, and Arf are molecular switches that are "off" when bound to GDP and "on" when bound to GTP
|
|
|
what activates the GTPase, and therefore hydrolyzes GTP to GDP, turning off the G proteins?
|
Guanine triphosphatase (GAPs)
|
|
|
What causes G proteins to release GDP and bind GTP, turning ON the G proteins?
|
Guanine nucleotide exchanger (GEFs)
|
|
|
Once the importin/Ran-GTP complex has transported back to the cytoplasm, what causes Ran to dissociate and thus begin a new cycle?
|
Ran-GAP causes GTP to hydrolyze and frees Ran to begin another cycle
|
|
|
What amino acids comprise the nuclear localization signals on cargo proteins being exported OUT of the nucleus?
|
Leucine
|
|
|
What is required to translocate cargo protiens out of the nucleus through the nucleporins?
|
Exportin is the passport to export OUT of the nucleus, which binds to Ran-GTP
|
|
|
What stimulates GTP hydrolysis after Ran-GTP coupled with exportin have translocated cargo proteins outside of the nucleus?
|
Ran-Gap hydrolyzes the GTP to GDP, releases the exported cargo
|
|
|
How are mRNAs transported across nuclear pore complexes?
|
They are transported as RNA-protein complexes (must have a protein buddy) heterogeneous nuclear ribonucleoproteins - hnRNPs
|
|
|
What mRNA's are recognized by the proteins and are thus allowed to export the nucleus?
|
Only mature, fully processed mRNAs are allowed out (5' cap, poly A tail, spliced)
|
|
|
Where do the hnRNP proteins bind the mature mRNA and why is this necessary to export them outside of the nucleus?
|
They bind the 5' cap and have nuclear export signals recognized by exportins (leucine-rich)
|
|
|
Where does translation on large mRNAs begin?
|
On the ribosomes on the surface of the outer nuclear membrane, which is continuous with the rER
|
|
|
Where are Ran-GDP and Ran-GTP and what maintains them?
|
ran-GDP in cytosol = Ran GAP (aoutside)
ran-GTP in nucleus = Ran GEF (eenside) |
|
|
Heterochromatin is compact and inactive while euchromatin is unwound, active chromatin
|
What is the difference between heterochromatin and euchromatin?
|
|
|
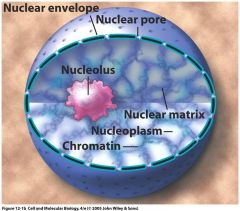
The nuclear envelope has 2 bilyers with nuclear pore complexes throughout. Heterchromain (active)is seen here
|

What comprises the nuclear envelope pictured here?
|
|
|
The NPC is composed of Glycoproteins that open in response to calcium
|
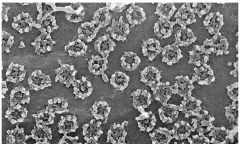
What sort of proteins make up the nuclear pore complexes and what causes them to open?
|
|
|
Import = K (Lysine), and R (arginine)
Export = Leucine |
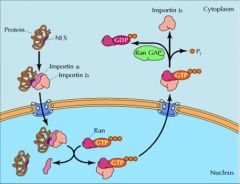
What Nuclear Localization Signals are used to import and export cargo proteins through the nuclear pore complexes to the nucleus?
|
|
|
How can viral genetic material pass into nucleus? Hepatitus B, Adenovirus and Herpes Simplex
|
Hepatitus B viral capsid can pass directly through NPC, while Adenovirus and Herpes Simplex must dock at cytoplamic side with the help of importins and then release viral DNA into nucleoplasm
|
|
|
What are the function of Lamin filaments?
|
These Intermediate filaments are localized to the nucleas, maintain cell shape, and protect the membrane
|
|
|
Where do lamins form in the nucleus? What functions do they serve?
|
Lamins are just under the inner nuclear membrane and provide strength, enable growth/membrane breakdown, and separate nuclear pores
|
|
|
What lamin is permanently anchored to the inner membrane?
|
Lamin B
|
|
|
During phosphrylation, what happens to the lamins in the nuclear membrane?
|
Lamins A and C are released as free dimers and Lamin B (farnesylated) stays anchored to the inner membrane
|
|
|
After the nucleas is dephosphorylated, what occurs to enabel reformation?
|
Lamin B forms vesicles that allow rapid reassembly
|
|
|
Where do the lamins bind to the inner nuclear membrane?
|
Lamin B binds to the the Lamin Binding Reception and Associated Proteins, while A/C bind to emerin
|
|
|
Why do sufferers of Emery-Dreifuss muscular dystrophy have cardiac conduction defects?
|
Lamins A/C fail to bind to emerin, thus the filaments supporting nucleas are weak and cannot support the contraction of the heart
|
|
|
What causes Lipodystrophy?
|
Lamin A/C defects cause lipodystrophy, which causes fat to accumulate in head and neck
|
|
|
What enzyme facilitates transcription and replication in the nuclear matrix?
|
Topoisomerase II
|
|
|
What substructures in the nucleas are involved in mRNA processing and recycling of snRNPs?
|
Speckles contain mRNA processing/splicing machinery, while Cajal bodies/GEMS recylcle snRNPs
|
|
|
What is the nucleolus?
|
The nucleolus are ribonucleoprotein factories that are at work durign interphase
|
|
|
What are the 3 subcompartments in the nucleolus?
|
Fibrillar centers
Dense Fibrillar components Granular components |
|
|
When are rRNA and tRNA synthesized in the cell cycle?
|
During interphase (G1, S, G2)the nucleolus assembles
|
|
|
Where is rRNA transciption initiated and where are rRNA transcripts modified within the nucleolus?
|
Transcription is initiated in the fibrillar centers of the nucleolus, and then the pre-rRNA transcripts are modified by snoRNPs in the Dense Fibrillar component region.
|
|
|
Lamins A, B, and C form the meshwork or "scaffold" under the inner membrane of the nuclear envelope
|
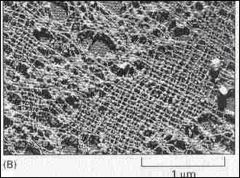
What is the fucntion of this struture within the nucleus?
|
|
|
The nucleolus is pictured on the left and assembles during interphase, while in mitosis, pictured on the right, protein synthesis is not occuring
|
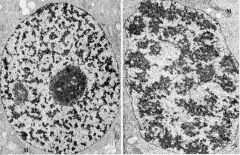
What structure is indicated on the left and what cell cycle phases are represented?
|
|
|
The lease dense areas are the sites of rRNA transciption initiation, the Fibrillar Centers.
|
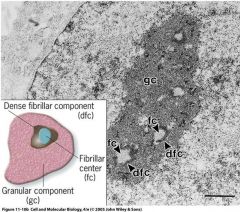
What is happening in the least dense areas in the nucleolus picture here?
|
|
|
RNA Pol I polymerizes rRNA The branches grow from rDNA in the middle and are capped by snoRNP terminal knobs on the ends
|
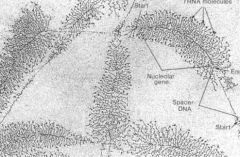
What is transcribing the growing rRNA chains below?
|
|
|
What is the last nucleolar subcompartment ribosomes continue through before they are exported through to the cytoplasm?
|
The Granular compartments
|
|
|
What ribosome protein subunits are assembled in the nucleolus?
|
45S pre-rRNA spliced into:
18S rRNA of 40S subunit 5.8S and 28S rRNA of 60S subunit |
|
|
What is different about 5.S rRNA transcription compared to the 28S and 5.8S rRNAs?
|
All of these rRNAs join the big (60s) subunit, but only 5S rRNA transciption occurs outside the nucleus byt RNA Pol III
|
|
|
What do snoRNAs and proteins combine to form? What do they do?
|
SnoRNAs (made by RNA Pol II) combine with proteins to form SnoRNPs. SnoRNPs modify rRNA in the Dense Fibrillar region of the nucleolus
|
|
|
A prominent nucleolus indicates that the cell is making lots of ribosomes and therefore lots of proteins. Pancreas, plasma, and developing blood cells all secrete proteins but CANCER cells will as well.
|
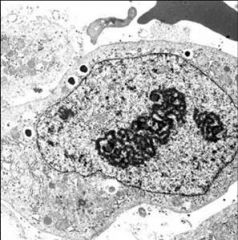
What does a prominent nucleolus, seen here, indicate? Why could this be a warning sign?
|
|
|
What are formed in the Endoplasmic Reticulum?
|
The proteins (rER) destined for secretion and the lipids (sER) that make up the membranes of organelles
|
|
|
What is continuous with the outer bilayer of the nucleous? Why is this advantageous?
|
Because the rER is continuous with the outer bilayer, transcription of the proteins can begin even before they have completely exited the ER
|
|
|
What stimulates rER and sER to increase?
|
The dynamic nature of the rER and sER are influenced by B cells (stimulated to secrete antibodies) and by cholesterol/lipid shortage
|
|
|
Once pre-mRNA has been processed and spliced, whre are the exons, transported through the NPC, translated?
|
The mRNA is translated on the free ribosomes in the cytosol or on ribosomes on the rER
|
|
|
What is Cotranslation Translocation?
|
As mRNA is translates into a polypeptide chain it can aso be translocated into the ER lumen
|
|
|
What forms the zip code for mRNA proteins once they emerge from the pore?
|
The amino acid ER signal sequence is recognized by the Signal Recognition Particle which bidn to the SRP receptor in the ER membrane to let the mRNA in
|
|
|
Where is the amino targeting sequence (ER Signal Sequence) located on the growing protein?
|
On the N-terminus of the growing polypetptide chain
|
|
|
After the Signal Recognition Particle has bound to the ER signal sequence on the growing polypeptide chain, where does the SRP receptor bring the ribosome?
|
The SRP binds to the SRP receptor in the ER membrane, which breaks off the Signal Recognition particle and pulls the ribosome over to the translocon
|
|
|
The Signal Recognition Particle binds to the ER signal sequence of the N-terminus of the growing chain. What activity does it bring?
|
SRP brings GTPase function with it
|
|
|
When is GTP hydrolyzed in the cotranslational translocation?
|
GTP is hydrolyzed by SRP and the SRP receptor. This powers the transfer of the polypeptide to the translocon and opens the gate
|
|
|
How many GTP are required for cotranslational translocation?
|
2 GTP = one to bring the polypeptide to the translocon site and 1 to open the ER gate and let it in
|
|
|
What 2 enzymes act on the new polypeptide chain as it enters the ER?
|
Bip, the lumenal ER chaperone helps the new protien fold and the "molecular ratchet" drives it into lumen using ATP
Signal Peptidase cleaves the new chain |
|
|
What is modified in the transfer of membrane-spanning proteins into the ER?
|
The hydrophobic regions of the translocating protein make it through the membrane and begin to fold in to an alpha-helix structure. This creates a stop-transfer signal and leaves the hydrophilc regions on either side of the membrane
|
|
|
How are integral membrane proteins formed in the ER?
|
The signal sequences are embedded within the protein, which makes interior loops. They also contain a signal anchor sequence, which signals the ER to bind the SRP and stop the transfer
|
|
|
What are the 2 molecular chaperones for the proteins as they fold in the ER?
|
Bip and Calnexin
|
|
|
What do the chaperone proteins do to misfolded proteins? What other things do they prevent the proteins from doing?
|
Bip, Calnexin prevent promiscuous interactions b/w proteins and bind the bad ones
|
|
|
What is added to the protein during cotranslational translocation in the ER?
|
An Oligosaccharide-Asparagine bond occurs that delivers the carbohydrate to the protein until a Dolichol lipid anchor is added.
|
|
|
What must occur before the Oligosaccharide can be flipped to the lumen of the ER?
|
The sugar tree grows on the outside until it sees the Asparagine and flips to the lumen, anchored by Dolichol.
|
|
|
After Asparagine has attached to the new protein on the inside of the ER lumen, what happens to the sugars?
|
Now it "trims" the tree by
removing the 4 n-linked sugars as the protein moves through the ER |
|
|
What processing of the oligosaccharide attached to the growing protein occurs in the Golgi?
|
O-linked glycosylation (attachment of sugar) attaches sugars to serine or threonine side groups
|
|
|
What process in the Golgi determines your ABO blood type?
|
O-linked glycosylation of the new protein in the Golgi is the same for glycolipids and glycoproteins and = blood type
|
|
|
Congenital Diseases of Glycosylation are what sort of disorders?
|
Autosomal Recessive
|
|
|
CDG1b, a European defect involving glycosylation, effects phosphomannose oligosaccharides in proteins in the ER. Symptoms and treatment?
|
GI bleeding (hepatic fibrosis), hypoglycemia, treat with mannose
|
|
|
Because the oxidizing environment in the ER encourages cysteine-cysteine bonds, what potential problem exists?
|
Wrong disulfide bonds can easily occur before the protein has folded into its final form
|
|
|
What trims the glycoprotein sugar tree?
|
Glucosidase I/II trim 2 glucoses and the last glucose binds to calnexin/Calreticulin
|
|
|
What do Calnexin/calreticulin do to the growing protein?
|
They sequester the protein, bind to Protein Disulfide Isomerase, and fold the protein
|
|
|
What removes the final glucose from the new glycoprotein and what puts it back if it is folded wrong?
|
Glucosidase II, Glucosyltransferase
|
|
|
What are the new glycoproteins anchored to?
|
GPI
|
|
|
What happens if a protein has a structural problem that chaperones cannot fix, such as exposed hydrophobic regions?
|
The protein is kicked back out throught the translocon, cytosolic ubiquitin attaches over and over (poly-ubiquination) and proteasomes degrade it down to amino acids
|
|
|
After the protein has undergone cotranslation/translocation and processing, where is it sorted and shipped?
|
Now it goes from the rER to the ERGIC, to the Cis-Golgi to the trans-Golgi, where it buds off
|
|
|
What vehicle do proteins take from the rER to the Golgi? What if they are going back?
|
Proteins going on to Golgi go in COP II-coated cars (anterograde), going back = COP I-coated cars (retrograde)
|
|
|
Which proteins are ER Resident proteins?
|
Proteins tagged as KDEL or KKXX
|
|
|
What aino acids are always on KKXX?
|
Lysine, Lysine, X, X
|
|
|
What falls off of the new proteins prior to fusion?
|
The coat protein (COP I, II) falls off, exposing SNAREs
|
|
|
Once the coating on the proteins fall off, what binding sites are exposed?
|
Binding sites for motor proteins to allow transport on microtubule/actin tracks
|
|
|
Where do KDEL proteins belong?
|
These are ER resident proteins, COP I brings them back
|
|
|
Why is cis-Golgi named cis?
|
Because it is next to the rER
|
|
|
How are the Vesicular transport andthe Cisternal maturation model different explanations for protiens movement through the Golgi?
|
Vesicular transport = vesicles in cis Golgi destined for the next layer gather, bud off
Cisternal Maturation = each level matures and becomes the next higher level |
|
|
Where do carbohydrate chains of glycoproteins/glycolipids ALWAYS face?
|
Outside the cell
|
|
|
Role of COP I and COP II
|
COP II: Sorting/taking secretory cargo out of ER
COP I: Retrograde transport from GOlgi back to ER |
|
|
What proteins enable the vesicel coat to be put ON or OFF the proteins?
|
GEF = Arf (COP I) and Sar (COP II)
|
|
|
What is the difference between constituitive vs. Regulated secretion vesicles?
|
Constituitive vesicles leave the trans golgi and go to their target membrane and bud while Regulated vesicles must wait for an external signal (usually Calcium)
|
|
|
Vesicle proteins with a mannose-6-phosphate tag are destined to be what?
|
Lysosomes
|
|
|
What do polarized cell signals along the regulated secretory pathway indicate?
|
Whether the protein should go to the apocal vs. basolateral membrane
|
|
|
During Constituitive and regulatory secretion of protein vesicle out the Golgi, what 2 results may occur from proteolysis?
|
Proteolysis (clipping) may = proalbumin - albumin, or proinsulin - inslin
|
|
|
When proteins are transferred to polarized cells, how may they be transported from one membrane to another?
|
Transcytosis
|
|
|
What happens if early endosomes do NOT have a transcytosis signal?
|
They end up in late endosomes and then lysosome
|
|
|
To what part of the membrane do GPI-linked proteins dircted?
|
The apical side
|
|
|
What happens to the GPI-anchored proteins on lipid rafts?
|
The lipid raftes (cholesterol, glycosphingolipids) are sites for signalling AND sorting
|
|
|
What stabilizes the lipid rafts in the trans-Golgi?
|
O and N glycans/lectins stabilize the lipid rafts
|
|
|
What do SNARE proteins do?
|
SNARE proteins provide specificity and catalyze vesicel fusion w/ mebranes
|
|
|
How do V-SNARE and T-SNARE proteins interact?
|
Vesicel SNARES interact with Target SNARES and form a complex
|
|
|
What regulate the docking of V-SNARES to T-SNARES?
|
Rab-GTP regulates the process, and GTP hydrolyzes to GDP upon docking
|
|
|
What binds to the V/T SNARE complex and allows the SNARE complex to disassemble and the SNAREs to be used in future vesicle transport?
|
NSF/SNAPS
|
|
|
What is another term for a synaptic v-SNARE and t-SNARES
|
Synaptobrevin, syntaxin
|
|
|
What allows synaptobrevin to bind syntaxin?
|
Rab-GTO and SNAP/NSF
|
|
|
What Calcium sensors on the synaptic vesicle membrane trigger the fusion?
|
Synaptotagmins (they interact with syntaxin on the plasma terminus of the axon
|
|
|
What do vesicles destined for axon terminals import?
|
Neurotransmitters from the sytosol
|
|
|
After docking on the synaptic cleft what triggers fusion of the vesicle and release of NT?
|
Rise in cytosolic Calcium
|
|
|
During what process are the synaptic vesicle that delivered the NT recovered? How often do they fill with NT?
|
The vesicle is recovered via endocytosis in clathrin-coated vesicles, which refill with NT and complete the cycle every 60 seconds
|
|
|
What causes Flaccid Paralysis? What do these toxins bind to?
|
Botulism toxins bind to SNAREs, so vesicles carring ACh cannot fuse to pre-synaptic membrane, preventing muscle contraction
|
|
|
What does tetanospasm cleave and what is the result?
|
Cleaves synaptobrevin (v-snare) and inhibits release of inhibitory neutrotransmitters glycine and GABA = constant muscle contraction!
|
|
|
How does black widow venom impact NT release?
|
It increases Calcium levels, stimulating exocytosis of acetylcholine NT
|

