![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
47 Cards in this Set
- Front
- Back
|
Myoglobin |
-Located in Sarcoplasm ( cytoplasm of multinecleate muscle cell)
|
|
|
Heme |
prosthetic group |
|
|
confirmational chanage in heme |
-pucker- hemoglobin bends out to bind to proximal his 02 pulls iron back to center from other face of heme -distal his pulls O2 away preventing oxidation |
|
|
Hemoglobin |
-2 alpha 2 beta heterotetramer -O2 binding can be measure photospectrometerically at 580nm |
|
|
Kd |
[P][L]/[P-L] |
|
|
Theta |
occupied sites/ total sites [P-L]/([P-L]+[P])= [L]/([L]+Kd) O2/(O2+Kd) pO2/(pO2+p50) |
|
|
Michaelis-Menten'Equaton |
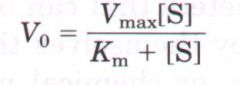
where V0 is the instantaneous change of concentration of Product over time |
|
|
Oxidoreductases |
remove hydrogen atoms from (oxidize) a donor molecule and concomitantly add hydrogen atoms to (reduce) an acceptor molecule. |
|
|
Transferases |
transfer a group of atoms (such as an acetyl moiety or a phosphoryl group) from one |
|
|
Hydrolases |
cleave a bond by adding the atoms of a water molecule across it. Such enzymes catalyze the hydrolysis of the anhydride bonds in all biopolymers, namely proteins (amide bonds), |
|
|
Lysases |
remove a group of atoms (like CO2 or H2O) from a substrate, leaving a double bond; or, |
|
|
Isomerases |
change the relative configuration of atoms within a molecule with respect to one another |
|
|
Ligases |
join two previously separate molecules together, using the energy derived from cleavage of |
|
|
Bohr effect |
Acidity and higher CO2 concentration causes Hb to release O2 -in muscles where CO2 and H+ concentrations are high, the hemoglobin will relase O2 to help |
|
|
Explain the saturation curve of Hb |
sigmoidal: When it starts to bond, the affinity increases, afterwards is it rate dependent then it decreases |
|
|
Dihydrofolate reductase |
When dUMP turns to DTMP which turns to DNA - DHFR turns NADPH+ H+ --> NADP |
|
|
Hallmarks of enzyme-mediated catalysis |
1. Tremendous rate enhancement 2. Occurs under physiological conditions 3. Precise reaction: highly selective for substrates; stereo-specific products 4. Subject to metabolic regulation |
|
|
How does an active site expedite a chemical reaction |
1. Entropy gain from substrates shedding water of solvation and displacing water bound in active site 2. Molecular complementary 3. H-bonds and salt bridges between groups on substrates and side chains in the active site are stronger in the absense of competition or shielding by water 4. Binding at the active site holds the substrates in close procimity 5. some binding energy expended to introduce strain into bonds into induced fit |
|
|
linear plot of Michaelis Menten eqn |

|
|
|
Kcat |
same as k2, turnover number per second |
|
|
Specificity constant |
Kcat/Km A good enzyme has a high Kcat and a low Km |
|
|
Reversible inhibitors (4 types) |
Do not covalently react with enzyme (1) Competitive (Transition state analogues) Vmax constant, Km --> alpha(Km) (2) Uncompetitive (Lowers Vmax, Lowers Km) (3) Mixed inhibition (binds both to enzyme and enzyme substate complex) EI <--> ESI as well, increases Km, decreases Vmax (4) Noncompetitive inhibitors (special case where Ki = Ki') Km doesn't change |
|
|
Progression from primary polypeptide to mature protein: alpha Chymotrypsin |
-Hydrophobic chain at beginning acts as "baggage ticket" to enter cell |
|
|
Km |
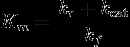
(kr+kcat)/(kf) kr=enzyme unbinding kcat=product formation kf=enzyme binding |
|
|
What is the molecular structure of Sarin |

|
|
|
Group specific reagents |

|
|
|
Reactive substrate analogs |

|
|
|
Suicide (Mechanism-based) inhibitors |
Nonreactive until substrate starts to react with it Catalysis generates the compound to make the enzyme become inactive |
|
|
Configuration of active site of chymotrypsin |
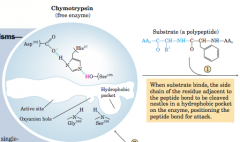
Asp102...His57-NH2... HO-Ser195 |
|
|
Acylation 1 |

|
|
|
Acylation 2 |

|
|
|
Deacylation 1 |

|
|
|
Deacylation 2 (final step of mechanism) |

|
|
|
Threonine products |
Hydro xyethyl _TP same enzyme uses 3 subsrates to create Ille , Val, Leu |
|
|
K allosteric enzyme |
small, sigmoidal curves adding substrate --> more active sites substate is an allosteric effector, makes binding to subrate easier. Inhibitor Km increases, substrate to the left vmax stays the same |
|
|
Confirmational preferences |

|
|
|
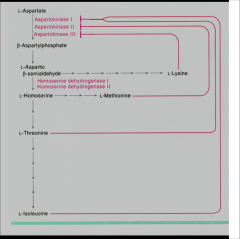
multiple isozymes subject to independent feedback inhibition (E-T) |
|
|
|
How T, I, and V are kept in balance |
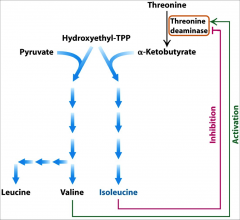
|
|
|
V allosteric enzyme |
Vmax changes but Km stays the same inactive state binds just as well as active state, but won't make product |
|
|
ATCase |
dodecomer with catalytic trimer and regulatory dimer stacked twice PALA is a bisubstate analog that can activate by conformational change in low concentrations like Hb, has a sigmoidal curve |
|
|
Enzyme regulation by reversible phosphorylation |

|
|
|
Name the three types of irreversible inhibitors |
-Reactive substrate analogues (Tosyl Phenylalanine Chloromethyl Ketone, Histidine of Chymotrypsin) -Group-specific reagents (Bromoacetol phosphate, reacts with glutamine) OR (DIPF, inactivates Acetylcholin esterase) -Suicide (mechanism-based) inhibitors |
|
|
Amino acids most likely to adopt alpha conformation |
Q, A, L, M, E, K, R, H |
|
|
Amino acids most likely to adopt Beta strand |
V, I, Y, C, W, F , T |
|
|
Amino acids most likely to adopt reverse turn |
G, N, P, S, D |
|
|
Assay for hemoglobin saturation |
Spectrophotometer-- oxygenated hemoglobin absorbes at 580 peak |
|
|
Y axis of hill eqn |
log (theta/1-theta) |

