![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
5 Cards in this Set
- Front
- Back
|
Draw the structure of ATP, ADP, and AMP
|
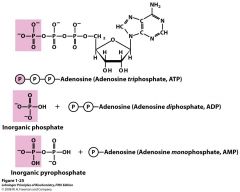
High energy bond b/w the phosphate, hydrolysis of high-energy bond releasing the stored energy and forming ADP and phosphate once again., energy use for an exergonic rxn.
|
|
|
First law of thermodynamics
|
Energy can be converted from one form to another, but cannot be destroyed.
*Energy of universe = energy of a system + energy of its surroundings “Something always happens at the expense of something else” |
|
|
Second law of thermodynamics
|
The entropy of an isolated system increases in an irreversible process, and remains unchanged in a reversible process. It can never decrease.
|
|
|
entropy
|
a thermodynamic quantity that expresses the degree of disorder or randomness in a system.
|
|
|
Is the reaction of ATP Hydrolysis thermodynamically spontaneous? if so for what reasons?
|
Yes, due to enthalpy (the internal energy of a system), entropy, and the rxn results in the solvation of the ATP
solvation = The process by which solvent (liquid) molecules surround and interact with solute (anything that dissolve in liq) ions or molecules |

