![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
42 Cards in this Set
- Front
- Back
|
Explain Protons |
- Found in the Nucleus of an atom. · - Each proton has a +1e charge, and a mass of one atomic mass unit (amu) - Atomic number (Z) of an element is equal to the amount of protons in an atom of an element |
|
|
Explain Neutrons |
- Neutral, have no charge · - Mass is only slightly larger than proton · - Together (proton, neutron) of the nucleus make up almost the entire mass of the atom. · - Every at has a mass number (A) which is the sum of protons and neutrons in the nucleus · - An atom can have varying amounts of neutrons, thus atoms always have the same atomic number but do not necessarily have the same mass number. · - Isotopes are atoms that share an atomic number but have different mass numbers · - The convention ^A_Z X is used to show the atomic number (Z) and the mass number (A) of atom X |
|
|
Size and charge of electrons |
· Move through space surrounding nucleus · Each electron has a charge equal in magnitude tothat of a proton, but negative (-1eor -e) · Mass is 1/2000 of a proton |
|
|
Explain electrons and distance from nucleus |
· Move around nucleus at varying distances, which correspond to levels of potential electric energy. (Further>near) |
|
|
Explain Electrostatic and gravitational forces on electrons |
· Because of their small mass, the electrostatic force of contraction betweenthe unlike charges of the proton an electron is far greater than thegravitational force of attraction based on their masses |
|
|
What determines the reactivity of an atom |
valence electrons |
|
|
what are valence electrons |
Electrons furthest away have the strongestinteractions with the environment and weakest with the nucleus, these arecalled Valence Electrons. |
|
|
Explain sharing and transferring of valence electrons |
· Sharing or transferring of valence electronsallows elements to fill their highest energy level to increase stability. |
|
|
What is the neutral state |
Neutralstate has equal numbers of protons and electrons |
|
|
What terms are synonyms for describing the heaviness of an element |
Atomicmass and mass number (A) are synonymous fordescribing the heaviness of an element. |
|
|
What is atomic weight |
- In nature, almost all elements exist as two or more isotopes. · -The weighted average of these different isotopes is referred to as the Atomic Weight and is the number on the periodic table. - Atomic weight represents both the mass of the “average” atom of that element in amu, and the mass of one mole of the element in grams. |
|
|
Explain Atomic Mass |
The atomic mass of an atom is nearly equal to its mass number, the sum of its protons and neutrons |
|
|
What are isotopes |
Isotopes differ in their number of neutrons and are reffered to by the name of the element followed by the mass number; ie carbon-12. |
|
|
What is a mole |
- Mole is a number of “things” (atoms, ions, molecules) equal to Avogadro’s number, NA=6.02 x 10^23 Eg. A carbon has an atomic weight of 12.0 amu, which means the average carbon has a moss of 12.0 amu. And 6.02 x 10^23 carbon atoms have a combined mass of 12 grams. |
|
|
What is given by Planck relation |
The energyof a quantum is given by the Planckrelation: E=hf h is aproportionally constant known as Planck’sconstant, equal to 6.626 x 10^-34 J x S· f isthe frequency of radiation |
|
|
Equation for the angular momentum of an electron orbiting a hydrogen nucleus |
L=nh/2π n is the principal quantum number, which can be any positive integer· his Planck’s constant Bohr then related the permitted angular momentumvalues to the energy of the electron to obtain E= -R_H/n^2 ----> Where Rh is the experimentally determined Rydberg unit of energy, equal to 2.18 x 10^-18J/electron |
|
|
What does the energy equation say? |
E= -R_H/n^2 Energyequation is saying is that the energy of an electron increases (becomes lessnegative) the farther out from the nucleus it is located (increasing n) |
|
|
What is the ground state |
TheGround State of an atom is the stateof lowest energy, in which all electrons are in the lowest possible orbitals.· MCAT Expertise: all systems tend towardminimal energy; thus on the MCAT, atoms of any element will generally exist inthe ground stat unless subjected to extremely high temperatures or irradiation. |
|
|
applications of the bohr model |
Useful for explaining the atomic emission andabsorption spectra of atoms. |
|
|
What is the equation for electromagnetic energy of photons |
E=hc/wavelength h is plack's constant c= speed of light in a vaccum (3x10^8 m/s) |
|
|
As electrons go from a lower energy level to a higher energy level they.... |
Get AHED Absorb light Higher potential Excited Distant (from the nucleus) |
|
|
Explain energy transitions |
Energy translations do not form a continuum, but rather are quantized to certain values. Thus the spectrum is composed of light at specified E --->Sometimes called a line spectrum. -Each element can have its electrons excited to different levels, each posses a unique atomic emission spectrum, which can be used to fingerprint an element. |
|
|
Explain The atomic emission spectrums |
-Lyman series is the group of hydrogen emission lines corresponding to transitions from energy levels n ≥ 2 to n=1. o - Levels of n≥3 to n=2 is the Balmer series, includes four wavelengths in the visible region o - Levels of n ≥ 4 to n=3 is the Pachen series · -----> Energy with a change in the principle quantum number from a higher initial ni value to a lower final value nf, is equal to the energy of the photon predicted by Planck’s quantum theory. |
|
|
What is Planck's quantum theory |
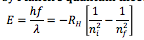
An equation that says: the energy emitted photon corresponds to the difference in energy between the higher-energy initial state the lower-energy final state |
|
|
Explain the atomic absorption spectra |
- When electron is excited to a higher energy level, it must absorb exactly the right amount of energy to make the transition · - Every element possesses a characteristic absorption spectrum. Wavelength of absorption correspond exactly to the wavelengths of emission. - Takeaway: each element has a characteristic set of energy levels. For electrons to move from a lower energy level they must absorb the right amount of energy (in the form of light) to do so. When they move down they emit the same amount of energy in the form of light |
|
|
What is the Heisenberguncertainty principle |
Heisenberguncertainty principle: It isimpossible to simultaneously determine, with perfect accuracy, the momentum andthe position of an electron |
|
|
Explain quantum numbers |
- Any electron in an atom can be completely described by four quantum numbers: n, l, m_l, and m_s. · - Pauli exclusion principle, no two electrons in a given atom can possess the same set of four quantum numbers · - Energy State describes the position and energy of an electron · - Value of n limits the Value of l, which in turn limits the value of ml. |
|
|
What is the Pauli exclusion principle |
- Pauli exclusion principle, no two electrons in a given atom can possess the same set of four quantum numbers · |
|
|
Explain the principle quantum number |
- First quantum number noted by n · - Used in bohr’s model and can take on any positive integer value · - Higher the value, higher the energy level and radius of the electrons shell · - W/in each shell there’s a capacity to hold a certain number of electrons = 2n^2 |
|
|
Explain the aximuthal quantum number |
-Also called the angular momentum quantum number· - Denoted by letter l· - Refers to the shape and number of subshells· - --> The possible range for l is 0 to (n-1)· - Spectroscopic Notation refers to the shorthand representation ofthe principle and azimuthal quantum numbers. The principle quantum numberremains a number, but the azimuthal quantum number is designated by the letter.· ---> L=0 subshell is called s; l=1 called p; l=2 called d; l=3 called f.· ---> An electron in the shell n=4 and subshell l=2 issaid to be the 4d subshell. |
|
|
Explain spectroscopic notation |
- Spectroscopic Notation refers to the shorthand representation of the principle and azimuthal quantum numbers. The principle quantum number remains a number, but the azimuthal quantum number is designated by the letter. · ---> L=0 subshell is called s; l=1 called p; l=2 called d; l=3 called f. · ---> An electron in the shell n=4 and subshell l=2 is said to be the 4d subshell. |
|
|
Explain the magnetic quantum number |
- Third number designated by m_l · - Specifies a particular orbital w/in a subshell where an electron is most likely to be found at a given moment in time · - Each orbital can hold a maximum of two electrons · - Possible values of ml are the integers between –l and +l, including 0 · MCAT: shapes of the orbitals in the d and f subshells are much more complex and their appearance wont be tested. · |
|
|
Explain the orbitals in subshells (Shape and number) |
-S has one orbital (0), are spherical · -P has 3 orbitals (-1 to +1), dumbbell shaped and aligh along the x-, y- and z-axes. · - D has five orbitals (-2 to +2) · - F has seven orbitals (-3 to +3) · Shape of orbitals are dependent on the subshell in which they are formed |
|
|
Explain the spin quantum number |
-Denoted by m_s · - Two spin orientations are +1/2 and -1/2 · -Whenever two electrons are in the same orbital they must have opposite spins and are referred to as being Paired · - Parallel spins refers to electrons in different orbitals with the same ms values |
|
|
Explain electron configuration |
-Pattern the subshells are filled - Use spectroscopic notation, first number denotes the principle energy level, letter designates the subshell, superscript gives the number of electrons in that subshell. · - Eg. 2p^4 indicated there are 4 elections in the second (p) subshell of the second principal energy level. · - Electrons fill from lower to higher energy subshells according to the Aufbau principle (building up principle) No need to memorize the order because of two rules 1) The n+l rule can be used to rank subshells by increasing energy. Rule states the lower the sum of the values of the first and second quantum numbers, n+l, the lower the energy of the subshell. · 2) If two subshells posses the same n+l value, the subshell with the lower n values has lower energy and will fill with electrons first |
|
|
Draw the order of electron filling |
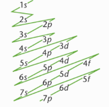
|
|
|
What is Hund's Rule |
- In subshells with more than one orbital (such as the 2p with three orbitals) the orbitals will fill according to hund’s rule. --Hund’s rule states that, within a given subshell orbitals are filled such that there are a maximum number of half-filled orbitals with parallel spins. ---> Like finding a seat on a bus, electrons would prefer their own seat (orbital) before being forced to double up with another electron. - Important to remember half-filled and fullyfilled orbitals have lower energies (higher stability) than other states. Thiscreates two notable exceptions often used on the MCAT: Chromium (and other elements in its groups) and copper (andother elements in its group). |
|
|
What are the notable exceptions often usedo n the MCAT for Hund's rule |
-Chromium (and other elements in its groups) and copper (and other elements inits group). -->Chromium(Z=24) should have the electron configuration [Ar] 4s^2 3d^4according to the rules. However moving one electron from the 4s subshell to the3d subshell allows the 3d subshell to be half filled: [Ar] 4s^1 3d^5(remember s subshells can hold 2 electrons and d can hold 10) - -> Copper(z=29) has the configuration [Ar]4s13d10 rather than[Ar]4s23d9. - Similar shifts c an be see with f subshells, butthey are never observed for the p subshell; the extra stability doesn’toutweigh the cost.· -The presence of paired or unpaired electronsaffects the chemical and magnetic properties of an atom or molecule. |
|
|
Explain valence electrons |
- Are the electrons in the outer most energy shell, are most easily removed and available for bonding. · - For elements in group IA and IIA (groups 1 and 2), only the highest s subshell electrons are valence electrons. · - For elements in group IIIA and VIIIA (groups 13 through 18), the highest s and p subshell electrons are valence electrons. · - For transition elements, the valence electrons are those in the highest s and d subshells, even though they have different principal quantum numbers. · - For the lanthanide and actinide series, the valence electrons are those in the highest s and f subshells, even though they have different principal quantum numbers. · - All elements in period three (Starting with sodium) and below may accept electrons into their d subshell, which allows them to hold more than eight electrons in their valence shells. This allows them to violate the octet rule. |
|
|
What are paramagnetic materials |
Materials composed of atoms with unpaired electrons will orient their spins in alignment with a magnetic field, and thus the material will be weakly attracted to the magnetic field. These materials are considered paramagnetic. |
|
|
What are diamagnetic materials |
Diamagneticmaterials consist only of atoms with paired electrons and are slightlyrepelled by a magnetic field. |
|
|
Valence Electrons |
- Are the electrons in the outer most energy shell, are most easily removed and available for bonding. · - For elements in group IA and IIA (groups 1 and 2), only the highest s subshell electrons are valence electrons. · - For elements in group IIIA and VIIIA (groups 13 through 18), the highest s and p subshell electrons are valence electrons. · - For transition elements, the valence electrons are those in the highest s and d subshells, even though they have different principal quantum numbers. · - For the lanthanide and actinide series, the valence electrons are those in the highest s and f subshells, even though they have different principal quantum numbers. · - All elements in period three (Starting with sodium) and below may accept electrons into their d subshell, which allows them to hold more than eight electrons in their valence shells. This allows them to violate the octet rule. |

