![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
11 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Volume |

the amount of space that matter occupies. |
|
|
|
Solid |

a state of matter that has a definite shape and definite volume. |
|
|
|
Liquid |

a state of matter that has NO definite shape and has a definite volume |
|
|
|
Gas |
a state of matter with NO definite shape or volume |

|
|
|
Physical Properties |
A characteristic of a pure substance that can be observed without changing it into another substance. |

|
|
|
Mass |
a measure of how much matter is in an object. |

|
|
|
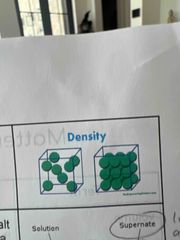
Density |
the measurement of how much mass of a substance is contained in a given volume. |
|
|
|
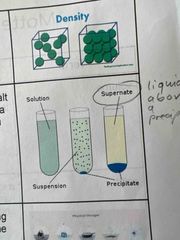
Solubility |
a measure of how much solute can dissolve in a given solvent at a given temperature. |
|
|
|
Physical change |
a change that alters the form or appearance of a material but does not make the material into another substance. |

|
|
|
Chemical change |
a change in which one or more substances combine or break apart to form new substances |

|
|
|
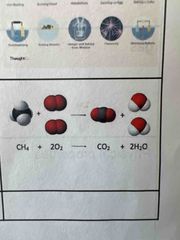
Law of conservation of mass |
the principle that the total amount of matter remains the same, regardless of any chemical or physical change. |
|

