![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
28 Cards in this Set
- Front
- Back
|
Phase Transformation Phases |
Nucleation and Growth |
|
|
Nucleation |
The appearance of very small particles/nuclei of a new phase which are capable of growing. Similar to like seed crystals. |
|
|
Growth |
Nuclei increase in size resulting in the disappearance of some/all parent phase. |
|
|
Equilibrium Fraction |
The point where transformation stops growth |
|
|
Types of Nucleation |
Homogeneous and heterogeneous |
|
|
Homogeneous Nucleation |
Nucleation occurs uniformally throughout parent phase. |
|
|
Heterogeneous Nucleation |
Nucleation occurring at structural inhomogeneities (container surfaces, impurities, dislocations, grain boundaries) |
|
|
Gibbs Free Energy |
Change in the energy within the system. |
|
|
Austenite |
A solid solution of carbon in a nonmagnetic form of iron, stable at high temperatures. It is a constituent of some forms of steel. |
|
|
Pearlite |
Two phase lamellar structure composed of alternating layers of ferrite and cementing. Harder and stronger than Spheroidite. |
|
|
Bainite |
Very fine and parallel needles of ferrite separated by elongated particles of cementite. Stronger and harder than both Pearlite and Spheroidite |
|
|
Spheroidite |
A ferrite matrix Weak and soft |
|
|
Cementite |
Spheroidal shaped particles |
|
|
Coring |
When a heated alloy cools in non-equalibrium conditions. The exterior solidifies before the interior. It also causes the centers of the grains to retain more of the higher melting temperature element. |
|
|
Eutectic Composition solidification |
Alloy forms a microstructure consisting of alternating layers of the two phases due to solidification atomic diffusion. Because of this layered configuration the diffusion path length for the atoms is a minimum. |
|
|
Variables which dictate the microstructure of an alloy |
Alloying elements present, the concentrations of these elements, the heat treatment of the alloy. |
|
|
Superheating and Supercooling |
Correspond respectively to heating or cooling above or below a phase transition temperature without the occurrence of the transformation. At the phase transition temperature, the driving force is not sufficient to cause the transformation. The driving force is enhanced during Superheating or supercooling. |
|
|
Force of the formation of Spheroidite |
The net reduction in ferrite-cementite phase boundary area. |
|
|
Fatigue Striations |
Microscopic features on a fatigue fracture surface which identify one propagation cycle of a fatigue crack. Seen through electron microscope. Used to identify fatigue fractures. Can be used to find the number of fatigue cycles. |
|
|
Benchmarks |
Macroscopic fatigue features marking an interruption of some sort in the fatigue cracking progress. |
|
|
Four measures to increase the fatigue resistance of an alloy |
Polish the surface to remove stress amplification sites Reduce number of internal defects by altering processing and fabrication Modify design to eliminate notches and sharp contour changes. Harden the outer surface of the structure by case hardening or shot peening |
|
|
Four Classifications of Steel, their properties and applications |
Low Carbon Steels: Nonresponsive to heat treatments, soft and weak, machine able and well able. Autobodies, structural shapes, pipelines, buildings, bridges, tin cans. Medium Carbon Steels: Heat Treatable, large combination of mechanical characteristics. Railway wheels and tracks, gears, crankshaft, machine parts. High Carbon Steels: Hard, Strong, Brittle. Chisels, hammers, knives, hacksaw blades High Alloy Steels: Hard and wear resistant: Resistant to corrosion in a large variety of environments. Cutting tools, drills, cutlery, food processing, surgical tools. |
|
|
reasons to use ferrous alloys |
iron ores exist in abudance Economical extraction, refining and fabrication techniques are available alloys may be tailored to have a wide range of properties. |
|
|
disadvantages of ferrous alloys |
susceptible to corrosion relatively high density relatively low electrical conductivities |
|
|
purpose of Alloying elements in tool steels |
the alloying elements in tool steels combine with the carbon to form very hard and wear resistant carbide compounds. |
|
|
why is gray iron brittle and weak in tension |
the tips of the graphite flakes act as points of stress concentration. |
|
|
gray vs malleable cast iron composition, microstructure, and mechanical characteristics. |
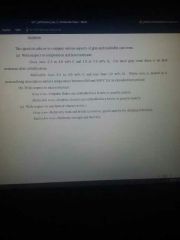
|
|
|
white vs nodular cast iron composition, microstructure, and mechanical characteristics. |
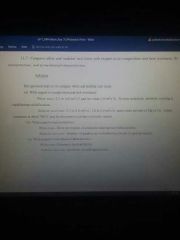
|

