![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
117 Cards in this Set
- Front
- Back
|
What is 'V' and what is 'Q'?
|
V is ventilation, the amount of air reaching the alveoli, given in L/min
Q is perfusion, the amount of blood reaching the alveoli, in L/min |
|
|
What is a normal gas exchange ratio?
|
5L of air breathed, 4L of blood perfused, so around 0.8
|
|
|
Does a normal V:Q ratio mean that air is oxygenated?
|
No. For example, you could have a blockage of one lung, and of the vein supplying that lung, so both blood flow and ventilation are reduced, meaning the ratio is preserved due to the normal functioning of the other lung.
|
|
|
Does all venous blood reach the lungs?
|
No, there are some R-L shunts - bronchiole veins, and some draining the L heart drain directly into the L side without undergoing perfusion.
|
|
|
What proportion of venous blood reaches the lungs?
|
98%
|
|
|
Give three examples of an abnormal R-L shunt
|
Collapsed lung
Consolidated lung (e.g. lobar pneumonia) Congenital defect (e.g. Fallot's Tetralogy) |
|
|
What type of shunt is caused by a septal defect of the atria or ventricles?
|
L-R. This does not cause a reduction in arterial PO2.
|
|
|
Given normal venous O2/CO2 content of 150ml/L and 520ml/L and normal arterial O2/CO2 content of 200ml/L and 480ml/L, what would the effect of a 20% R-L shunt be?
|
O2 = (0.8 x 200) + (0.2 x 150) = 190ml/L
CO2 = (0.8 x 480) + (0.2 x 520) = 488ml/L Due to shape of curves, small fall in O2 content = big change in PO2 (13.3kPa to 9kPa), where as a small rise in CO2 content has virtually no effect on PCO2 (still around <6kPa) |
|
|
What physiological effects would you see in a 20% R-L shunt?
|
Raised CO2 stimulates chemoreceptors to increase breathing rate
Increased ventilation blows off excess CO2 but you pick up no extra O2, so PO2 low, PCO2 normal. |
|
|
Would breathing oxygen help in a 20% R-L shunt?
|
Not to raise PO2. Blood that can be perfused is almost at saturation so won't take extra O2 on, and the shunted blood cannot be perfused.
|
|
|
If a region has less blood flow/perfusion to the amount of ventilation, how does it behave?
|
Like alveolar dead space.
|
|
|
If a region has less ventilation than is required for the amount of perfusion, how does it behave?
|
Like a R-L shunt.
|
|
|
An area has high ventilation, but low perfusion, how does it behave?
|
Alveolar dead space.
|
|
|
An area has good perfusion, but low ventilation, how does it behave?
|
R-L shunt.
|
|
|
Do areas with high V:Q and low V:Q cancel each other out, and why?
|
No. Areas with a high V:Q are low perfusion areas, so although blood is well ventilated, there is little of it. Areas with a low V:Q carry low O2 content, but perfusion is good so more blood passes through here. The result is more under-perfused blood than normally perfused.
|
|
|
Why does a R-L shunt often present with low PCO2?
|
High initial PCO2 is detected by central chemoreceptors which increase the breathing rate. Faster breathing removes excess PCO2 and possibly overcompensates, hence low PCO2. When the patient gets tired, PCO2 is likely to rise (dangerous!)
|
|
|
How do patients with R-L shunt and mixed V:Q mismatch respond to O2 enriched air?
|
In a pure R-L shunt, no real change. But in mixed V:Q mismatch you would see a marked improvement as O2 exchange would increase in poorly ventilated areas.
|
|
|
What is the effect of gravity on V:Q ratios in the normal lung when stood upright?
|
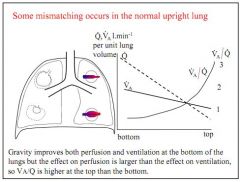
Gravity improves both ventilation and perfusion in the bottom of the normal lung, but perfusion more than ventilation, so V:Q ratio is higher in the top of the lung.
|
|
|
How does the lung respond to hypoxia and when might this be unhelpful?
|
Pulmonary vasoconstriction in areas of low ventilation helps maintain V:Q ratio. Not helpful in global hypoxia, e.g. altitude
|
|
|
How can you assess V:Q in a patient?
|
Isotope scan of lungs
Measure alveolar dead space and use Bohr and shunt equation Look at arterial/alveolar PO2 gradient. |
|
|
Of all the mechanisms for low PO2, which is the only one likely to lead to a rise in PCO2?
|
Hypoventilation, as in all others normal ventilation is likely to result in a low PCO2.
|
|
|
Define respiratory failure
|
When the body cannot maintain arterial blood gases within certain limits:
PO2 <8kPa PCO2 >6.7kPa |
|
|
What are the two types of respiratory failure?
|
Type 1 - Hypoxaemic = failure of gas exchange, causing low PO2 with low or normal PCO2
Type 2 - Hypercapnic = failure of ventilation, causing low PO2 with high PCO2 |
|
|
Give some example of causes of acute and chronic respiratory failures.
|
Acute - asthma, pneumonia, oedema, embolus
Chronic - COPD, lung fibrosis |
|
|
Why does type 1 respiratory failure not cause a rise in PCO2?
|
At the alveolar capillary exchange surface, CO2 diffuses around 20 times better than O2, so CO2 is rarely withheld.
|
|
|
Why does type 2 respiratory failure result in high PCO2?
|
Ventilation is directly proportional to PCO2, so in type 2 failure there is a failure of ventilation and therefore a failure to blow off CO2.
|
|
|
Given the relationship between PCO2 and V, what happens to PCO2 if V doubles/halves?
|
When ventilation halves, PCO2 doubles, and vice versa.
|
|
|
List the 5 mechanisms which can lead to arterial hypoxia.
|
Low inspired PO2 (e.g. altitude)
Diffusion impairment (e.g. fibrosis/inflammation) V:Q mismatch R-L shunt (e.g. collapse, consolidation, fallot's tetralogy) Hypoventilation (e.g. trauma) |
|
|
What are the two main causes of hypoventilation?
|
1. Mechanical/anatomical - e.g. inadequate chest wall movement, nerve damage, kyphoscoliosis
2. Failure of breathing control, e.g. sleep apnoea or COPD loss of sensitivity to PCO2. |
|
|
Briefly describe the neural and chemical control of breathing.
|
Brainstem neurones create pattern, higher centres (chemo/mechanoreceptors) modulate pattern
Brainstem chemoreceptors sense changes in PCO2 via changes in CSF pH. |
|
|
Where are the brainstem chemoreceptors located?
|
Ventrolateral medulla, by cranial nerves 9 and 10.
|
|
|
Patients with type 2 respiratory failure lose sensitivity to PCO2 in their central chemoreceptors, what drives respiration in these patients?
|
Hypoxia. Low PO2 drives greater respiration.
|
|
|
What are the clinical effects of acute hypoxia?
|
Confusion (CNS impairment), fits, coma, cyanosis, arrhythmia, pulmonary vasoconstriction.
|
|
|
What are the clinical effects of acute hypercapnia?
|
Respiratory acidosis, vasodilation, bounding pulse, low BP, confusion, coma, hand twitch, headaches.
|
|
|
What are the clinical signs of chronic hypoxia?
|
Polycythemia (increased RBC count), flushed, cyanosed, right ventricular hypertrophy, renal fluid retention, peripheral oedema.
|
|
|
What are the clinical signs of chronic hypercapnia?
|
Increased HCO3- production in kidneys to normalise pH, reduced respiratory drive for given PCO2.
|
|
|
What causes cyanosis?
|
High concentration of deoxyhaemoglobin.
|
|
|
What are the classic appearances of patients with type 1 and type 2 respiratory failure and why?
|

Type 2 - Blue bloaters, overweight, cyanosed, oedema, high PCO2
Type 1 - Pink puffers, underweight, hyperventilating, red faces, thin lips |
|
|
How would you treat type 2 respiratory failure?
|
High flow, low O2 concentration mask, small O2 delivery
|
|
|
How would you treat type 1 respiratory failure?
|
Low flow, high O2 concentration mask.
|
|
|
How is the lower respiratory tract kept sterile?
|
Filtration in upper airways, and mucus coating collects bugs and foreign bodies, which is then swept out of lung and dissolved in stomach.
|
|
|
Is the common cold bacterial or viral?
|
Viral, of mixed origin (rhinovirus, coronavirus etc)
|
|
|
What types of viral infection cause a pharyngitis?
|
Colds and flu, glandular fever (EBV), herpes simplex, coxsackie virus (hand, foot and mouth).
|
|
|
What bacterial infections cause a pharyngitis?
|
Strep pyogenes (Group A Strep throat infection)
|
|
|
What are the characteristics of group A strep?
|
Gram positive cocci, haemolyse blood agar plates.
|
|
|
Can diptheria cause pharyngitis?
|
Yes. Commonly infects tonsils, larynx and pharynx. Rarely infects nose or skin.
|
|
|
Why is epiglottitis an emergency in young children?
|
Swollen epiglottis can block airway very quickly.
|
|
|
Describe influenza.
|
RNA virus, three types A, B and C. Expresses Haemaglutinin and Neuraminidase antigens
|
|
|
List some upper respiratory tract infections.
|
Pharyngitis, colds and flu, laryngitis, sinusitis, otitis media, laryngotracheobronchitis (flu types)
|
|
|
What is bronchitis?
|
Lower respiratory tract infection.
|
|
|
Is bronchitis viral or bacterial?
|
Mostly viral causes - in chronic cases, a bacterial infection (strep pneumoniae, haemophilus, mycoplasma bacterium) exacerbate condition.
|
|
|
What are the causes of pneumonias?
|
Strep pneumoniae
Heamophilus influenzae Mycoplasma pneumoniae Chlamydia Influenza TB Legionella Staph Aureus |
|
|
Can pneumonia be acquired in hospital?
|
Yes. Commonly coliforms like klebsiella, E Coli.
|
|
|
TB is a cause of pneumonia, but does it spread elsewhere?
|
Yes, 10-15% of patients will develop a disseminated TB.
|
|
|
Where do inhaled pathogens originate?
|
Exogenous - other patients, animals, environment (e.g. Legionella)
Endogenous - from other body part, Upper Respiratory Tract, stomach (esp on antacids), blood. |
|
|
What are the risk factors for a respiratory infection?
|
Age, recent infection (low immunity), major surgery.
|
|
|
What are the four strategies for treating infection?
|
Do nothing - symptomatic relief (e.g. painkiller, fluids)
Surgery to drain e.g. abcess Boost immunity/avoid immunosuppression Antibiotics |
|
|
What is an anti-biotic?
|
Natural substance produced by an organism which kills or inhibits bacteria. Has no effect on viruses.
|
|
|
What is an anti-microbial?
|
Natural or synthetic substance effective against microbes - anti-septic, anti-fungal, anti-parasite.
|
|
|
Why are clinicians reluctant to prescribe antibiotics?
|
1. Resistance develops
2. Cost 3. Side effects |
|
|
What are some of the current issues with overprescribing?
|
Course too long
Wide spectrum when narrow spectrum would do Too much given IV, with increased risk of IV infection Misused as prophylaxis (more doses given than necessary) |
|
|
What is MIC and MBC?
|
MIC - Minimum Inhibitory Concentration, amount of antibiotic needed to inhibit growth
MBC - Minimum Bactericidal Concentration, amount needed to kill bacteria |
|
|
What are the main categories of anti-biotics?
|
Beta-lactams (e.g. Penicillin),
Macrolides (e.g. erythromycin) Chloramphenicol Sulphonamides, trimethoprim Aminoglycosides e.g. gentamicin Metronidazole Tetracyclines e.g. oxytetracycline Quinolones e.g. ciprofloxacin Glycopeptides e.g vancomycin |
|
|
How do beta-lactams work?
|
They bind penicillin binding proteins on the cell membrane of bacteria, they make the wall osmotically unstable and prevent the bacteria from dividing as it grows. Eventually, the bacteria gets so big it dies.
|
|
|
How do macrolides work?
|
Inhibit protein sythnthesis in the bacteria.
|
|
|
How does anti-biotic resistance develop?
|
Rapid evolution due to genetic mutations, especially if anti-biotics over prescribed.
|
|
|
Describe the mechanism by which a bacteria might use an enzyme to destroy penicillin prescribed to kill it?
|
Beta-lactamase is released by the bacteria, attacking the beta-lactam ring on the anti-biotic.
|
|
|
Where are enzymes produced by bacteria which can prevent effectiveness of antibiotics?
|
Staph Aureus (extracellularly), or some gram negatives from within the periplasmic space.
|
|
|
How might a bacteria prevent the action of an aminoglycoside like gentamicin?
|
AAC - Aminoglycoside-modifying enzyme.
|
|
|
How does strep pneumonaie develop resistance?
|
Alters the Penicillin Binding Protein (PBP) to become less susceptible.
|
|
|
How does MRSA form resistance to flucloxacillin and methicilin?
|

Alters Penicillin Binding Protein
|
|
|
How does Vancomycin-resistant enterococci form resistance to vancomycin?
|
Changes the cell wall penta-peptide.
|
|
|
Why is penicillin not active against pseudomonas and E Coli?
|
It cannot penetrate the cell wall.
|
|
|
How does pseudomonas prevent gentamicin from penetrating its outer cell wall?
|
Develops mutated porins which prevent the anti-pseudomoas biotic entering the outer cell wall
|
|
|
How do some resistant coliforms survive after antibiotics have penetrated the cell wall?
|
They pump out the antibiotic (Efflux).
|
|
|
How is resistance detected?
|
Failure to respond or erratic drug compliance
Carry out standard agar/broth tests Look for specific resistant enzymes/PCR for resistant genes |
|
|
What are the three main mechanisms for resistance spread?
|
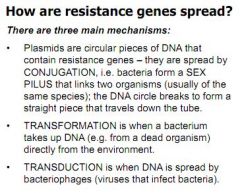
Conjugation - Bacteria form a sex pilus
Transformation - Bacteria takes up DNA from a dead organism Transduction - DNA is spread by bacteriophages (viruses that infect bacteria) |
|
|
Where do resistance genes come from?
|
Not from mutations - these are too specific. Thought to come from the organism which produced the antibiotic, which then adapts to create new enzymes against other antibiotics.
|
|
|
What is the name for the nucliec acid core and capsid protein coat?
|
Nucleocapsid
|
|
|
What is influenza and what causes it?
|
Zoonosis caused by orthomyoxoviridae, single strand RNA with a lipid envelope containing two glycoproteins, Hemaglutinin and Neauraminidase.
|
|
|
What are the major antigenic determinants of influenza?
|
Hemaglutinin and Neuraminidase
|
|
|
How many subtypes of influenza A would you expect?
|
15 known HA and 9 NA variants.
|
|
|
Which animal is the reservoir for all flu types?
|
Wild birds.
|
|
|
What are the two types of antigenic shift which account for variation in flu viruses?
|
Antigenic shift - minor changes in RNA copying, accounts for seasonal mutations
Antigenic drift - sudden and major genomic change, accounts for pandemic |
|
|
Describe antigenic drift.
|
RNA replication error accumulation, renders immune response less effective
|
|
|
Describe antigenic shift.
|
Reassortment of genetic segments, effectively creates a new virus which immune responses are not primed to encounter. Doesn't happen in influenza B or C.
|
|
|
How is a flu pandemic diagnosed?
|
Nucleic acid detection - PCR
|
|
|
How is the flu vaccine made?
|
3 components are combined to make a flu subunit, grown in unfertilised eggs, then inactivated and purified. Up to 70% antigenic similarity to circulating virus.
|
|
|
Who should get the flu vaccine?
|
At risk patients, young, old, health workers, sick.
|
|
|
How do Relenza and Tamiflu work?
|
Inhibits neuraminidase, preventing progeny virus from budding - needs neuraminidase to break bond of sialic acid.
|
|
|
What is a normal resting core temperature?
|
36.5 and 37.5 degrees c.
|
|
|
What is hypothermia?
|
<36degrees c
|
|
|
What is hyperthermia?
|
>40degrees c
|
|
|
What are the best ways to gauge core temperature?
|
Ear and mouth (can underestimate core), bum, GI pill.
|
|
|
List five factors affecting heat storage.
|
Metabolic production, convection, conduction, radiation, evaporation
|
|
|
By what process does heat move from the core to the skin in the blood?
|
Convection
|
|
|
By what process does heat move through the skin itself and into the air layer immediately next to the skin?
|
Conduction
|
|
|
How does the body control conductive, convective and radiant heat gain/loss?
|
Controlling blood flow through the skin via arterio/venous shunts.
|
|
|
How can we increase metabolic heat production?
|
Exercise/shivering
|
|
|
How can we increase evaporative heat loss?
|
Sweating.
|
|
|
What is the thermoneutral zone?
|
The ambient temperature at which the naked body can maintain core temperature by varying skin blood flow. Around 27-31 degrees c.
|
|
|
What happens to superficial veins in the cold?
|
Constriction, blood returns via deep veins.
|
|
|
Which nerves control arteriovenous anastamoses?
|
SNS.
|
|
|
What would a reduced/increased sympathetic discharge to the arteriovenous anastamoses cause?
|
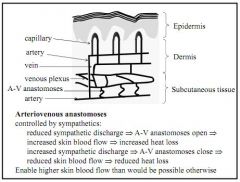
Reduced discharge opens anastamoses, increasing skin flow/heat loss
Increased discharge closes anastamoses, decreasing skin flow/heat loss |
|
|
Which fibres stimulate sweat glands?
|
Cholinergic (parasympathetic)
|
|
|
Is sweat hypotonic, isotonic or hypertonic to plasma?
|
Hypotonic, around 10-60mmol/L
|
|
|
Shivering is known to increase metabolic heat production as much as 5 fold, is this also true in neonates?
|
No.
|
|
|
Which part of the hypothalamus contains heat sensitive areas?
|
Anterior (pre-optic)
|
|
|
Which part of the hypothalamus contains cold sensitive areas?
|
Posterior
|
|
|
Is there overlap in the temperature range of the cold and warm receptors?
|
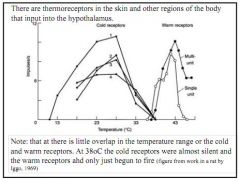
Almost none. At 38degree the cold are silent.
|
|
|
How does surface temperature affect the set point for sweating and shivering?
|
When skin is warm, set point for sweating is lower. When skin is cold, set point for sweating is raised.
When skin is cold, temperature for shivering rises, and when skin is hot, temperature for shivering is reduced. |
|
|
What is the impact of pyrogens like IL-6 on the set point for sweating?
|
Pyrogens raise the set point, you feel cold even though body is building up heat more without losing it through evaporation.
|
|
|
What is acclimation?
|
Adaptive change in response to artificial stimuli
|
|
|
What is acclimatisation?
|
Adaptive change in response to natural exposure
|
|
|
What is adaptation?
|
Adaptive change due to genetics/habituation
|
|
|
How do we acclimatise to heat?
|
Lower sweating threshold (sweat easier)
Increase sweat rate Lower heart rate Vasodilation at lowere core temp Take around 3-14 days to occur |
|
|
How do we acclimatise to cold?
|

Some local effects but not well understood - we increase set point for peripheral vasodilation, which reduces maintenance of core temp, and improves sleep and manual dexterity
|

