![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
85 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What are the three ways that mitochondrial genetics differs from mendelian genetics?
|
1.) Heteroplasmy / Threshold Effect
2.) Maternal Inheritance 3.) Mitotic Segregation |
Things that make you go HMM...
|
|
|
Intercellular and intergenerational mtDNA transmission follows what principle?
|
Principle of population genetics.
|
STFU Young. You don't need a hint for this one.
|
|
|
Why do children of heteroplasmy mothers have widely varying proportions of mtDNA genotypes?
|
Due to random partitioning of mitochondria during expansion of oogonial population.
|
How does mitochondria (not mtDNA) segregate in oogenesis?
|
|
|
Define Homoplasmy
|
The presence of one type of mtDNA in each cell
|
"Homo" and "plasmy"
|
|
|
Define Heteroplasmy
|
The presence of more than one genetically distince mtDNA in each cell.
|
"Hetero" and "plasmy"
|
|
|
For all mtDNA mutations, the clinical expressions are dependent on what three factors?
|
1.) Heteroplasmy
2.) Threshold Effect 3.) Tissue Distribution |
HTT
|
|
|
How does heteroplasmy effect clinical expression?
|
Based on the abundance of mutant mtDNA
|
Ratio
|
|
|
How does the Threshold Effect factor in to clinical expression?
|
Based on the vulnerability of each tissue to impaired oxidative metabolism
|
OX
|
|
|
How does tissue distribution affect the clinical expression?
|
Based on the distribution of mutant mtDNA in tissue.
|
?
|
|
|
asdfsaf
|
asfd
|
afdasf
|
|
|
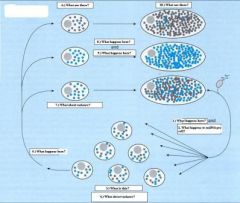
1.) Fertilization
2.) Decrease in mtDNA copy number per cell 3.) Primordial germ cells 4.) Low intercellular variance 5.) Segregation 6.) Primary oocytes 7.) High intercellular variance 8.) Oocyte maturation 9.) mtDNA amplification 10.) Mature Oocyte |
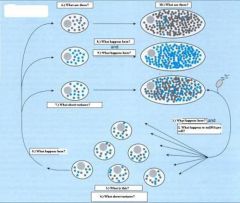
_
|
Should be obvious
|
|
|
Tissues most dependent (most affected) by OXPHOS function. X 4
|
CNS, Muscle, Vision, and Hearing.
|
|
|
|
Give an example of a pleiotropy mutation?
|
A3243G mutation on the tRNA-leu gene.
|
|
|
|
Give four variable expressions for the pleiotropic mutation of A3243G mutation on the tRNA-leu gene.
|
1.) MELAS (Mitochondrial Encephelomyopathy with Lactic Acidosis and Stroke episodes)
2.) Diabetes / Deafness 3.) CPEO (Chronic Progressive External Opthalmoplegia) 4.) Cardiomyopathy |
|
|
|
Give an example of three overlapping phenotypes resulting from a mtDNA deletion mutation.
|
1.) Kearns/Sayres Syndrome
2.) Pearson Syndrom 3.) PEO (Progressive External Opthalmoplegia) |
Kiss the pear and pee it out.
|
|
|
Mitochondrial DNA deletions occur how?
|
de novo
|
latin
|
|
|
Define nondisjunction
|
Failure of homologous chromosomes (meiosis I / mitosis) or sister chromatids (meiosis II) to properly separate in to different progeny cells.
|
|
|
|
Define uniparental disomy (UPD)
|
Disomic cell line containing two chromosomes of a given kind from only one parent
|
|
|
|
Two types of uniparental disomy (UPD)
|
1.) Isodisomy
2.) Heterodisomy |
|
|
|
Define Isodisomy
|
Two of the same (homolog) chromosomes present in a cell line
|
|
|
|
Define Heterodisomy
|
Two, different chromosomes present from only one parent in a cell line
|
|
|
|
List four possible causes of disease seen with UPD.
|
1.) AR disorder from one document parent carrier
2.) X-linked disorder transmitted from father to son (paternal heterodisomy) 3.) X-linked disorder expressed from father to daughter in homozygous form (paternal isodisomy) 4.) Involving genetic imprinting. |
|
|
|
1.) Chromosomes fail to separate to daughter cells
2.) Meiosis I (Drama!) 3.) Meiosis II (No drama) 4.) Biparental inheritance 5.) Biparental inheritance 6.) Uniparental heterodisomy 7.) Uniparental isodisomy resulting from chromosome duplication. |
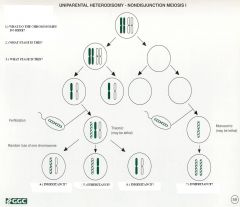
Nondisjunction in Meiosis I
|
|
|
|
1.) Meiosis I
2.) Meiosis II 3.) Chromosomes fail to separate properly to daughter cells 4.) Biparental inheritance 5.) Biparental inheritance 6.) Uniparental isodisomy 7.) Uniparental isodisomy resulting from chrosome duplication |
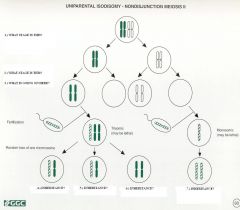
Nondisjunction in Meiosis II
|
|
|
|
Define Genetic imprinting
|
Parent of Origin specific silencing of a subset of mammalian genes
|
|
|
|
Define epigenetic
|
A set of reversible heritable gene function that occur without a change in the DNA sequence.
|
|
|
|
What type of phenomenon is imprinting?
|
Epigenetic
|
|
|
|
Imprinting involves what two mechanisms?
|
1.) Allele specific methylation AND/OR
2.) Histone modification and alteration of chromatin structure. |
|
|
|
When are imprints "erased?"
|
In between generations where new imprints established based on gender of germ line.
|
|
|
|
T/F - Is genetic imprinting tissue specific?
|
T - Imprinting occurs in some tissues and biallelic in others.
|
|
|
|
When is genetic imprinting regulated?
|
Regulated early during embryonic development, but biallelic in adults.
|
|
|
|
When are imprinted genes expressed?
|
Expressed during embryo and placenta development, as well as prenatal and postnatal brain.
|
|
|
|
With the occurence of imprinted genes, paternally expressed genes promote what?
|
Growth
|
|
|
|
With the occurence of imprinted genes, maternally expressed genes promote what?
|
Growth regulation
|
|
|
|
List the 5 paternally expressed (maternally imprinted) genes on 15q-11-q13
|
1.) ZNF127
2.) IPW 3.) NDN 4.) SNURP / SNRPN 5.) MAGEL2 |
After drinking zinfadel, I pee with an indian, snurp snurpin, and magel too.
|
|
|
List the maternally expressed (paternally imprinted) gene on 15q-11-q13
|
UBE3A
|
Women, you be 3 assholes
|
|
|
A deletion in what gene causes either PW or AS
|
15q-11-q13
|
|
|
|
What genetic cause is the most common cause of Prader-Willi?
|
Paternally deleted genes on 15q-11-q13
|
|
|
|
What genetic cause is the most common cause of Angelman Syndrome?
|
Maternally deleted genes on 15q-11-q13
|
|
|
|
How can UPD play a role in Prader-Willi
|
A patient can have PW if they obtained both pairs of the chromosome maternally
|
|
|
|
How can UPD play a role in Angelman's Syndrome
|
A patient can have AS if they obtained both pairs of the chromosome paternally
|
|
|
|
Dynamic mutations defined
|
A mechanism of a mutation that is caused by the expansion of a repeated sequence in novel genes
|
|
|
|
Common Trinucleotide Repeat Expansion Mutations X 2
|
1.) CAG
2.) CGG |
|
|
|
Trinucleotide Repeat Expansion mutations are usually found in which part of the gene (general)? X 2
|
1.) In genes that regulate transcription factors
2.) In genes that regulate development |
|
|
|
What can be anticipated in trinucleotide repeat expansion mutations during successive generations? X 3
|
1.) Earlier onset of disease.
2.) Increasing severity. 3.) Increasing length of repeats |
|
|
|
In what type of diseases do trinucleotide repeats expand with paternal transmission?
|
CAG repeat diseases (Type I)
|
|
|
|
In what type of diseases do you see expansion of trinucleotide repeats associated with maternal transmission?
|
1.) Fragile X Syndrome (CGG)
2.) Myotonic dystrophy (CTG) 3.) Friedreich Ataxia (GAA) |
|
|
|
Where do you find Type I trinucleotide repeat expansion mutations?
|
Coding regions
|
|
|
|
Where do you find Type II trinucleotide repeat expansion mutations?
|
Noncoding regions
|
|
|
|
Type I trinucleotide repeat expansion mutations have what type of molecular and genetic character?
|
Genetic: CAG mutation in coding region
Molecular: Mutant protein usually has long polyglutamine tracts. |
|
|
|
Symptoms of Type I trinucleotide repeat expansion mutations.
|
Neurodegenerative disorder with neuronal loss in brain, brainstem, and spinal cord.
|
|
|
|
Define Gain of Function mutation and where it is usually seen in?
|
Mutation on gene creates new gene product that has a new and abnormal function.
Type I trinucleotide repeat expansion disorder Seen in Type I trinucleotide repeat expansion mutation |
|
|
|
What type of transmission is usually seen in Type I trinucleotide repeat expansion mutation
|
Autosomal Dominant except for SBMA (Spinobulbar muscular atrophy which is X-linked.
|
|
|
|
When do you see the greatest expansion in Type I trinucleotide repeat expansion mutation?
|
Father to daughter transmission.
|
|
|
|
Huntington Disease is what type of disorder?
|
Trinucleotide Repeat Expansion Type I
|
|
|
|
Genetically, what causes Huntington's Disease?
(type of repeat and chromosome #) |
CAG repeats on Chromosome 4
|
|
|
|
What kind of transmission type is Huntington's?
|
Autosomal Dominant
|
|
|
|
What can be said about the mutation rate of Huntington's?
|
One of the lowest mutation rates in humans.
|
|
|
|
Symptoms of Type II Trinucleotide Repeat Expansion.
|
Variety of clinical features other than neurodegeneration.
|
|
|
|
Penetrance of Fragile X syndrome.
|
Males: 80%
Females: 30% |
|
|
|
Inheritance character of fragile X with mental retardation.
|
Single most common inherited cause of mental retardation
|
|
|
|
Uniqueness of female transmitted Fragile X syndrome.
|
Only time when permutation can become full blown mutations
|
|
|
|
Where can we find the location of the Fragile X Syndrome gene?
|
FMR1 (on the distal end of Xq) has CGG repeats in the 5' end of the untranslated gene.
|
|
|
|
T/F - Is mental retardation associated with Fragile X permutation
|
NO.
|
|
|
|
What magnitude of repeats exist in the permutation state of Fragile X syndrome
|
59-200 CGG repeats
|
|
|
|
Unique character of male transmittance in Fragile X.
|
Males can not transfer to daughters, but those daughters can have affected sons.
|
|
|
|
Mechanism leading to Trinucleotide Repeat Expansion
|
Mechanism uncertain, but believed that slipped mispairing mediated by repeats during DNA synthesis causes extra repeats
|
|
|
|
Describe a mechanism used to maintain repeat integrity against trinucleotide repeat expansion disorders.
|
In FMR1 alleles, the AGG triplet interrupts the CGG region every 9-10 repeats, thus preventing slippage.
|
|
|
|
LHON stands for what?
|
Leber Hereditary Optic Neuropathy
|
|
|
|
What magnitude, type, and nature of mtDNA mutations cause LHON.
|
18 missense mutations of the homoplasmic kind in mitochondrial DNA.
|
|
|
|
How many primary location sites for mutatations can be attributed to LHON?
|
90% of those affected have mutations on three sites.
|
|
|
|
Describe the mutational load of LHON.
|
Mitochondrial, leukocyte mutational load over 75% leads to affected individuals.
|
|
|
|
Penetrance of LHON.
|
Males 40%
Females 10% |
|
|
|
What does MERRF stand for?
|
mitochondrial Myopathic myclonic Epilepsy Ragged Red Fiber on muscle biopsy
|
|
|
|
Where does the mutation for MERRF occur?
|
On the mitochondrial tRNA-lys gene
|
|
|
|
How many places of mutations are associated with MERRF?
|
90% of affecteds have mutations on four gene locations.
|
|
|
|
What type of mitochondrial genetics exist in MERRF patients?
|
Heteroplasmic with variable expression.
|
|
|
|
What type of stains are exhibited in MERRF and what does each show?
|
1.) Modified Gomori trichrome stain - shows ragged red fibers.
2.) Cytochrome Oxidase stain - shows lack of cytochrome oxidase in muscle fiber. |
|
|
|
In what tissues can we find mutations for MERRF typically?
|
All of them especially the WBC
|
|
|
|
Individuals with a few symptoms of MERRF or asymptomatic maternal relatives show what degree of mutation?
|
Undetectable mutation in mtDNA from leukocytes, but detectable in other tissues.
|
|
|
|
Critical factors that affect MERRF children X 2.
|
1.) Mutational load
2.) Tissue distribution |
|
|
|
MERRF recurrence risk in males and females.
|
Males: cannot pass it
Females: can pass it to all children |
|
|
|
Describe the treatments of mitochondrial disorders.
|
1.) Supportive
2.) Coenzyme Q10/ldebenone 3.) Riboflavin |
|
|
|
Describe the benefits of Coenzyme Q10/ldebenone in mitochondrial disorder
|
Subjective benefits.
1.) Bypass block with artificial electron acceptor 2.) Minimize free radical damage. |
|
|
|
Describe the benefits of Riboflavin in mitochondrial disorder.
|
Enhances residual activity in Complex I and Complex II deficiency.
|
|

