![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
60 Cards in this Set
- Front
- Back
|
Explain partial pressure (Dalton's law) with an example.
|
Dalton's Law says the partial pressure (P) of a gas in a mixture is the pressure it would exert if it occupied the volume alone. Air pressure at sea level is 101kPa. Oxygen composition in air is ~21%, so PO2 in air at sea level is 101 x 0.21 = ~21kPa
|
|
|
What effect does altitude have on PO2?
|

Barometric air pressure at altitude is much less than at sea level. At the top of Everest for example, air pressure is 33kPa. O2 content is the same (21%), so PO2 on Everest is 33 x 0.21 = ~7kPa
|
|
|
Explain the movement of a gas from liquid to gas phases using Henry's law.
|

Henry's law says the solubility of a gas in a liquid at a given temperature depends on the partial pressure of that gas above the liquid. So movement is dependent on partial pressure, not just the concentration in liquid. Partial pressure depends on solubility and content of gas in liquid.
|
|
|
What two factors affect the pressure of water vapour in the air around us?
|
Temperature and saturation.
|
|
|
Air is 100% saturated as it enters the lungs, the water vapour pressure is 6.3kPa. What is the PO2 of moistened air in the trachea?
|
Barometric pressure = 101kPa
Water vapour pressure = 6.3kPa 101 - 6.3 x 21% = 20kPa |
|
|
What is a typical PAO2 (pressure of oxygen in alveolar gas) and what factors determine it?
|
13.5kPa. Pressure of O2 in trachea (taking into account water vapour) less pressure of CO2 being exchanged.
|
|
|
What drives diffusion of O2 from the alveoli into the blood?
|
The pressure difference - it is high in alveolar gas, and low in deoxygenated blood from the right ventricle.
|
|
|
At what point does the PAO2 equal that in pulmonary blood?
|
About 1/3rd of the way along.
|
|
|
What factors affect the rate of diffusion across a membrane?
|
Area and thickness, and the pressure difference.
|
|
|
Why is carbon monoxide useful for studying the effects of factors on gas transfer?
|

Haemoglobin has 240x affinity for CO over O2. Hence when inspired, it immediately is absorbed, and PCO in pulmonary blood is assumed to be zero. This means that any CO expired was not capable of transfer.
|
|
|
What is transfer factor?
|
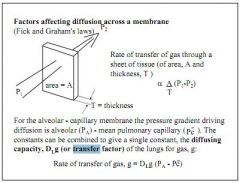
Carbon monoxide diffusion capacity.
|
|
|
What factors reduce ability to transfer CO across the alveolar membrane?
|
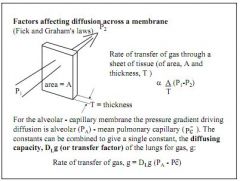
Reduced surface area - emphysema, lung resection
Thicker membrane - inflammation, oedema Reduced haemoglobin - anaemia |
|
|
Which factors increase ability to transfer CO across the alveolar membrane?
|

Increased blood volume - exercise
Polycythemia. |
|
|
How does PO2 affect tissue perfusion?
|
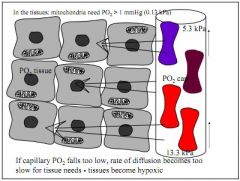
PO2 is essential for tissue perfusion, mitochondria require 0.1kPa to receive O2. If PO2 drops, tissue become hypoxic.
|
|
|
Is O2 soluble in blood, what is the resting O2 consumption, and how much blood flow would be required to supply body if O2 was only carried in solution?
|
O2 is slightly soluble in blood, at normal PO2 of 13kPa, there is 0.003ml O2 in each ml of blood.
Resting O2 required is 250ml/min, so if O2 was only dissolved in blood, you'd need 83L of blood per minute |
|
|
How many molecules of O2 are carried in the haem group?
|
4
|
|
|
What is the oxygen capacity of normal blood?
|
200ml/L
|
|
|
What is the oxygen content of pulmonary venous blood?
|
~100% saturated (99.9%)
|
|
|
What is the oxygen content of blood returning to the heart (mixed venous)?
|
75% saturated.
|
|
|
What does the oxygen haemoglobin dissociation curve explain about oxygen content, saturation, and PO2?
|
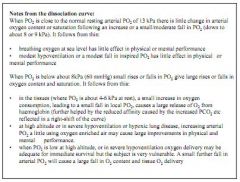
At normal PO2 (13.3kPa) blood is 100% saturated at 200ml/L
Mixed venous blood, 75% sat, content 150ml/L, has a PO2 of 5.3kPa On Everest, where PO2 is 7kPa, you would expect sats of around 80% |
|
|
The curve is fairly flat from around 9kPa, why is this important?
|
Above ~9kPa you can get sats of close to 100%, this means that changes in PO2 from 13.3kPa down to around 8kPa will have little physiological effect on the body.
|
|
|
The curve is steep between 0kPa and 8kPa, why is this relevant?
|
Below 8kPa, the curve falls away sharply. This means that below 8kPa you will see a rapid drop in O2 saturation, causing hypoxia.
|
|
|
What factors would shift the oxygen-haemoglobin dissociation curve to the left and what effect does that shift have on tissue oxygen demand?
|
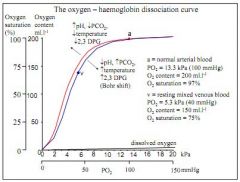
Rise in pH (alkalosis), reduction in PCO2 (e.g.hyperventilation), reduction in temperature (hypothermia), reduction in levels of DPG
|
|
|
What factors would sift the oxygen-haemoglobin dissociation curve to the right, and what effect does that shift have on tissue O2 demand?
|
Drop in pH (acidosis), increase in PCO2 (e.g.hypoventilation), increase in termperature (hyperthermia), rise in levels of DPG
|
|
|
What is DPG?
|
2,3 Disphosphoglycerate (DPG) is a metabolite created by red blood cells in glycolysis which has a high affinity for haemoglobin. It competes with O2 for binding sites, so high levels raise O2 requirements, and low levels reduce them.
|
|
|
Why do temperature, pH and PCO2 affect haemoglobin's affinity for O2?
|
They alter the structure of haemoglobin.
|
|
|
Describe the factors which influence the dissociation of O2 from haemoglobin in the tissues.
|
Oxygenated blood arrives in tissues with low PO2, this concentration gradient facilitates diffusion of O2 from haemoglobin (high PO2) to the tissues (lower PO2). The tissues have a high PCO2, and the haemoglobin low PCO2, driving gaseous exchange. Tissue temperature (raised) and pH (raised, esp in exercise) will also reduce haemoglobins affinity for O2.
|
|
|
Describe the factors influencing association of O2 in the lungs.
|
Deoxygenated blood arrives in lungs, with low PO2 and high PCO2. Blood has a high PO2 and low PCO2, driving gaseous exchange. Lower temperature in the lungs, higher pH, and low PCO2 all give haemoglobin a higher affinity for O2.
|
|
|
How does the curve vary in anaemia and CO poisoning?
|
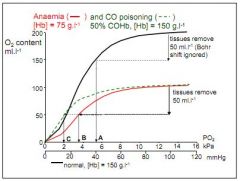
In CO poisoning there is competition for binding between CO and O2, 50% COHb causes drastic reduction in PO2 after tissue has removed oxygen. Anaemia is similar, but due to less [Hb] (50%) rather than competition for binding.
|
|
|
How does O2 dissociation differ in the foetus?
|
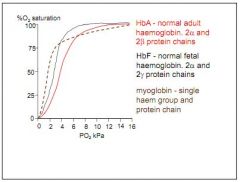
Foetus carries haemoglobin F (HbF), with 2-alpha and 2-gamma subunits. This produces high saturation at very low PO2 (steeper curve) 100% at around 7-8kPa.
|
|
|
How does O2 dissociation differ in myoglobin?
|
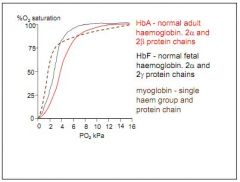
Very steep curve at low kPa, 75% at 2-3kPa, but trails off.
|
|
|
What is cyanosis indicative of?
|
Hypoxia - blue tinge due to high concentration of deoxy Hb.
|
|
|
What is peripheral cyanosis?
|
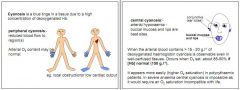
Reduced blood flow to the regions, arterial O2 content may be normal.
|
|
|
What is central cyanosis, and where is it observed?
|
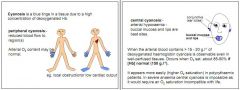
Mouth and lips, ear lobes. Occurs when O2 sats are 75-80%.
|
|
|
At what level do mitchondria require PO2 to be at to function?
|
0.13kPa.
|
|
|
What is normal tissue PO2?
|
2-5kPa.
|
|
|
What is the PCO2 in normal arterial blood?
|
5.3kPa
|
|
|
What are the two reactions in red blood cells relating to carbonic acid?
|
CO2 and H2O are converted to carbonic acid H2CO3
H2CO3 is converted to H+ and bicarbonate HCO3- |
|
|
Which enzyme in red blood cells facilitates the conversion of CO2 and H2O to carbonic acid H2CO3 and what is it's effect?
|
Carbonic anhydrase - speed up reaction (normally slow in plasma, v quick in RBCs)
|
|
|
What facilitates the conversion of H2CO3 to H+ and HCO3-?
|
Buffering of H+ ions by haemoglobin.
|
|
|
What happens to bicarbonate formed in RBCs?
|
Diffuses out down it's concentration gradient in exchange of Cl- (Chlorine shift)
|
|
|
What is the Chlorine shift?
|
Diffusion of bicarbonate out of red blood cells in exchange for Cl-.
|
|
|
What is the Haldane effect?
|

At any given PCO2, the quantity of CO2 carried is always greater in deoxygenated blood than oxygenated blood.
|
|
|
What are the two reasons for the Haldane effect?
|

The Haldane effect says that for any given PCO2, the amount of CO2 carried is always greater in deoxygenated blood than oxygenated. This is due to:
Hb forms carbamino compound much easier when deoxygenated Hb is a better buffer when deoxygenated |
|
|
What three mechanisms account for CO2 carriage?
|

1. HCO3- (bicarbonate) - 60%
2. Carbamino compounds - 30% 3. Dissolved CO2 - 10% |
|
|
What is the effect of the shape of the CO2 dissociation curve on arterial CO2 content?
|

It is more linear, so breathing out will greatly reduce PCO2 content, and not breathing out so much will greatly increase PCO2 content.
|
|
|
If normal arterial PCO2 is 5.3kPa, what is mixed venous PCO2?
|
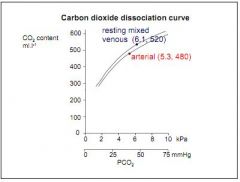
6.1kPa.
|
|
|
Why is the difference between arterial PCO2 (5.3kPa) and mixed venous PCO2 (6.1kPa) much less than the respective PO2 (13.3kPa, 5.3kPa)?
|

Due to the three mechanisms of CO2 uptake, blood has a much higher affinity for CO2 than O2.
|
|
|
Why is there a slightly greater difference between arterial and mixed venous O2 content than arterial venous CO2 content (O2 = 50ml/L, CO2 = 40ml/L)?
|
Because slightly more O2 is consumed than CO2 produced.
|
|
|
What factors influence arterial PCO2?
|
Any CO2 left in the artery after passing through the lungs is due to a difference between the amount we are producing, and the amount we are able to exhale.
|
|
|
What is hypercapnia?
|
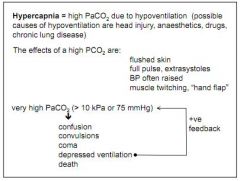
High PCO2. Results from hypoventilation (head injury, lung disease)
|
|
|
What are the effects of hypercapnia?
|
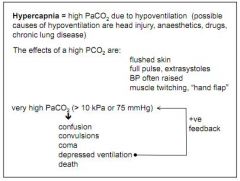
Flushed skin, raised BP, strong pulse, muscle twitching... If very high, coma, death.
|
|
|
What is hypocapnia?
|
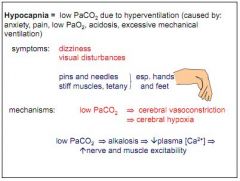
Low PCO2. Due to hyperventilation (anxiety, pain, low PO2, acidosis).
|
|
|
What are the effects of hypocapnia?
|
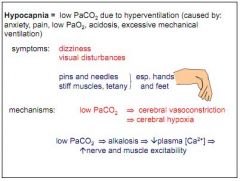
Dizziness, visual disturbances, pins and needles, tetany.
|
|
|
Why does hypocapnia cause tetany?
|
Reduced PCO2 leads to alkalosis, leads to low plasma Ca2+, causing twitches.
|
|
|
What 2 factors are said to influence short term behavioural change?
|
Information and fear of consequences of not changing behaviour.
|
|
|
In the health belief model, what 5 factors are said to influence someone to take a health action?
|
Vulnerability, severity, benefit of action, barriers to change, and cue to action.
|
|
|
What factors are said to influence behaviour in a reasoned action model?
|
Intentions, which are influenced by our attitude to the behaviour, and the subjective norm (based on attitudes of important others and now motivated we are to comply with those attitudes).
|
|
|
What additional factor is added in the theory of planned behaviour?
|
Self efficacy - our confidence that we can change our behaviour.
|
|
|
What are the two phases in Schwarzers model of change in health behaviour?
|
1. Motivation - self efficacy, outcome expectancy, threat/intention
2. Action - planning, behaviour and situation. |

