![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
97 Cards in this Set
- Front
- Back
|
what properties make up a biological cell membrane?
|
closed boundaries (sheet-like structures)
composed of lipids and proteins with varying ranges lipids have both hydro-phobic and philic moieties proteins serve many functions (pumps, channels, receptors, enzymes) everything is held together noncovalently two sides are assymetrical and 2-D fluid structures polarized (inside -60mV) |
|
|
how thick is a typical membrane?
|
60 A to 100 A
(6 nm to 10 nm) |
|
|
what is a fatty acid?
|
gives membrane hydrophobic quality
long hydrocarbon chains with varying lengths and degrees of unsaturation with a COO- on the end. |
|
|
C18:0
C18:1 C18:2 C18:3 name these bad boys |
C18:0 octadecanOIC ACID
C18:1 octadecENOIC ACID C18:2 octadecaDIENOIC ACID C18:3 octadecaTRIENOIC ACID |
|
|
Where does the numbering start on a fatty acid?
|
at carboxy terminus
C2 is a C3 is B terminal methyl is Omega |
|
|
what does cis^9 tell you naming wise?
Why is Omega 3 fatty acid confusing from a naming and counting standpoint? |
there is a double bond that is cis between 9 and 10
Omega 3 is confusing because you are counting starting from the Omega (distal) end of the thing and the double bond is then starting at 3 to 4. |
|
|
At pH 7, what form are fatty acids found in?
|
ionized form
palmitate (not palmitic acid) oleate (not oleic acid) refer to them as their carboxylate forms |
|
|
C14:0
|
Myristate
n-Tetradecanoate CH3(CH2)12COO- |
|
|
C16:0
|
Palmitate
n-Hexadecanoate CH3(CH2)14COO- |
|
|
C18:0
|
Stearate
n-Octadecanoate CH3(CH2)16COO- |
|
|
C20:0
|
Arachadecaonate
n-Eicosanoate CH3(CH2)18COO- |
|
|
C16:1
|
Palmitoleate
cis-^9-Hexadecenoate CH3(CH2)5CH==CH(CH2)7COO- |
|
|
C18:1
|
Oleate
cis-^9-Octadecenoate CH3(CH2)7CH==CH(CH2)7COO- |
|
|
C18:2
|
Linoleate
cis, cis-^9, ^12- Octadecadienoate CH3(CH2)4(CH==CHCH2)2(CH)6COO- |
|
|
C18:3
|
Linoleate
all cis -^9, ^12, ^15-Octadecatrienoate CH3CH2(CH==CHCH2)3(CH2)6COO- |
|
|
C20:4
|
Arachidonate
all cis-^5, ^8, ^11, ^14-Eicosatetraenoate CH3(CH2)4(CH==CHCH2)4(CH2)2COO- |
|
|
What is the typical biological range of Carbons on a fatty acid?
|
14-24 (usually even #)
16-18 most common |
|
|
What impact does higher degrees of unsaturation have on a fatty acid?
|
lowers the melting point
becomes more fluid |
|
|
what are phospholipids?
|
water insoluable, but soluable in organic solvents.
serve as hydrophobic and hydrophilic interacter for messaging, transducting and fuel |
|
|
describe a phospholipid
|
1 or more fatty acid
platform for fatty acid attachment (glycerol or sphingosine) phosphate alcohol on the phosphate |
|
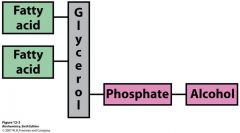
What is this?
|
phospholipid
|
|
|
Describe phosphatidate
|
simplest phosphoglyceride
3-Carbon glycerol platform with C-1 and C-2 esterified to fatty acid carboxyl groups C-3 is esterified to a phosphate group |
|
|
What are the 2 fatty acids on a phosphoglyceride typically?
|
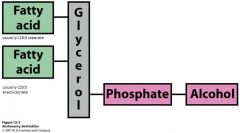
C1 is stearate (C18:0)
C2 is arachidonate (C20:0) |
|
|
what are the major phosphoglycerides?
|
derived from phosphatidate
add either Ser ethanolamine choline glycerol inositol with an ester bond to the phosphate and hydroxl of alchohol |
|
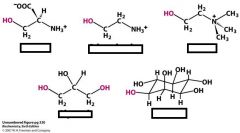
name these bad boys and memorize their functional groups
|
Ser
ethanolamine choline (3 methyls on the amine) glycerol inositol |
|
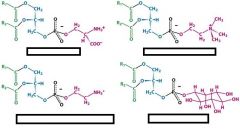
name these phosphoglycerides
|
Phosphatidylserine (PS)
Phosphatidylcholine (PC) Phosphatidylethanolamine (PE) Phosphatidylinositol (PI) |
|
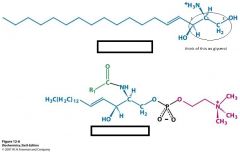
Name these structures
|
sphingosine
sphingomyelin |
|
|
where is the fatty acid attached to in sphingomyelin?
|
the amine of C2
|
|
|
what is a glycolipid?
|
sugar-containing lipids. Like sphingomyelin it is platformed of off sphingosine.
It is different from sphingomyelin by having a sugar attached to the primary hydroxyl of sphingosine |
|
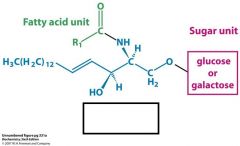
How is this lipid different from sphingomyelin?
|
cerebroside
glycolipid |
|
|
What are gangliosides?
Where are the sugars located with regard to the cell? |
more complex glycolipids
branched chain of up to 7 sugar residues sugars of glycolipids are always extracellular |
|
|
Describe cholesterol.
|
steroid with 4 linked hydrocarbon rings.
on one end is a hydrocarbon tail and a hydroxyl group on the other end. |
|
|
What is the orientation of cholesterol in the cell membrane?
|
parallel to phospholipids because the hydroxyl tail interacts with the proximal phospholipid head
|
|
|
cholesterol is found in all membranes of every kind of life form. verdad o falso?
|
False. Prokaryotes do not have cholesterol.
|
|
|
How are archea membranes unique?
|
non-polar chains attached to glycerol backbone
glycerol stereochemistry is inverted compared to phosphatidate alkyl chains are branched and saturated due to ether linkage they are resistant to hydrolysis and due to alkyl chains resistant to oxidation lipids help archea withstand high temps, high salt, low PH |
|
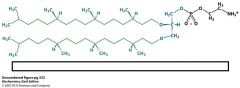
what is this structure and why is it unique?
|
archea lipid
branched, saturated alkyl chains with ether linkage inverted stereochem of glycerol |
|
|
what is an amphipathic molecule?
|
molecule with both hydrophilic and hydrophobic moieties
circle likes water, green tails hate water |
|
|
what is a micelle?
|
small globular structure <200A (20nm) in diameter
|
|
|
what is a lipid bilayer?
|
2 sheets of lipids.
Tails interact with eachother while the heads face out and interact with water. middle part is now a permeable barrier can form more extensive dimensions (1mm) over the micelle config. (those fatty acids won't fit in the middle) |
|
|
Give an example of a molecule that readily forms a micelle?
|
sodium palmitate
(only has one single fatty acid chain) |
|
|
describe the forces that make for lipid bilayers forming rapidly and spontaneously.
|
hydrophobic tail forces and van der waals forces between the hydrocarbon tails
hydrophilic forces of water interacting with heads (electrostatic and H bonding) |
|
|
what does cooperative structure mean?
|
many reinforcing, noncovalent interactions
makes lipid bilayers extensive, compartmentalized and self-sealing |
|
|
what is a liposome?
|
the creation of a lipid vessicle with an aqueous center
important because it allows us to study membrane permeability and to deliver drugs in the aqueous bit |
|
|
how do you form a liposome?
|
put PC in aq medium and sonicate
vessicles are uniform, spherical and about (50nm) in diameter |
|
|
describe molecular trapping.
|
first you make the liposomes with GLY in the vessicle.
Then you can observe how the GLY gets out and determine the lipid bilayers permeability to GLY You can also insert proteins using a detergent to see how permeability is effected |
|
|
what is doxil?
|
long-acting liposomal version of doxorubicin used to treat a variety of cancers
greater efficacy and lower cardiotoxicity |
|
|
What is Lipoplatin?
|
liposomal cisplatin 110 nm
treat karposi's sarcoma and multiple myeloma reduced nephrotoxicity, peripheral neuropathy, ototoxicity, myelotoxicity, and less nausea and vomiting |
|
|
How is the permeability of a lipid bilayer different for water and Na or K?
|
lipid bilayers are relatively impermeable to ions and polar molecules
But water, with its small size, abundance and lack of charge goes through easily |
|
|
describe how a small molecule might make "break on through, to the other side"
|
solvates in water, dissolves in hydrocarbon core of membrane, diffuses on through and gets re-solvated on the other side.
So those that easily replace their coordination shell are more quickly permeable |
|
|
Are ions more or less permeable than glucose?
Is Glucose more or less permeable than indole? |
ions less permeable than glucose
glucose less permeable than indole? |
|
|
Why is Tryptophan less permeable than indole?
|
indole lacks ionic groups--more energetically favorable to pass through the hydrophilic center of membrane
|
|
|
describe the relationship between membrane lipids and proteins
|
protiens transport chemicals and info across a membrane
membrane lipids create the appropriate environment for the action of these proteins |
|
|
what is the concentration of proteins for an electrically insulated environment like a nerve cell membrane?
|
18% proteins in myelin and those proteins will be found in the synapses
|
|
|
what is the concentration of proteins in a metabollically active membrane?
|
50% proteins in plasma membranes
pumps channels receptors and enzymes |
|
|
What concentration of proteins make up an energy transducing membrane?
|
inner mitochondrial membrane is a whopping 75% proteins.
Not as much lipids but a lot of cardiolipin |
|
|
what are the 3 ways proteins can be associated with membranes?
|
peripherally
integrally half and half (bottom half of protein has series of a helices that interact with hydrophobic middle of membrane) |
|
|
how are peripheral membrane proteins attached to membrane?
|
electrostatic and H bonded to lipid heads
bind to integral proteins hydrophobic fatty acid chain attaches to lipid bilayer |
|
|
How are integral proteins anchored in membrane?
|
They interact extensively with the hydrocarbon middle tails
|
|
|
What are 2 types of structures for integral membrane proteins?
|
a helices
B strands |
|
|
describe bacteriorhodopsin
|
7 perpendicular a helices span 45 A width of cell membrane
H+ gradient uses light energy to pump H+ out and synthesize ATP a helics are hydrophobic with non polar residues sticking out |
|
|
what are the most common structural motif in membrane spanning proteins?
|
a-helices
|
|
|
what is a porin?
|
channel protein
single antiparallel B-sheet which folds in a way to make a cylindrical channel The outside surface is nonpolar The inside surface is hydrophilic and water filled |
|
|
Where are porins commonly found?
|
E. coli
|
|
|
How is the compatible structure of a porin created?
|
alternating hydrophobic and hydrophilic residues that are h-bonded diagonally.
|
|
|
draw the structure for arachidonate
|
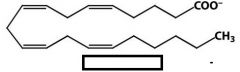
|
|
|
what is prostaglandin H2 synthase-1 and what does it do?
|
an enzyme that catalyzes the conversion of arachidonic acid into prostaglandin H2
Initiates COX-1 (cyclooxygenase rxn) (perioxidase rxn) |
|
|
what does prostaglandin H2 do in the body?
|
promotes inflammation and modulates gastric acid secretion
|
|
|
how does prostaglandin H2 differ synthase-1 differ from bacteriorhodopsin?
|
it has a-helices, but they are on the bottom of the protein and their hydrophobic amino acid side chains are what is imbedded in the membrane
|
|
|
What is thromboxane A2?
|
prothrombolitic
activation and aggregation of platelets causes vasoconstriction BAD aspirin works for keeping blood thinner by irreversibly inhibiting COX-1 and thus the formation of Prostaglandin H2 and thus TXA2 |
|
|
What is PGI2 (prostacyclin)
|
eicosanoid
decreases platelet aggregation causes vasodilation GOOD |
|
|
Is prostaglandin H2 synthase 1 an integral or peripheral protein?
|
integral, even though it is not fully imbedded.
Requires organic solvent or detergent to isolate that sucker. |
|
|
which residue inside the COX-1 protein channel gets the inhibiting aspirin acetyl group?
|
Ser 530
|
|
|
What are 2 important feature of how proteins interact with membranes?
|
1). parts of protein that interact with hydrophobic part of membrane are coated with non-polar amino acids side chains and parts interacting with aq part are hydrophilic
2). imbedded structures are quite regular participate in H bonding which are difficult to sever |
|
|
name the anchors for
palmitoyl farnesyl GPI |
thioester
C-terminus C-terminus |
|
|
name the 10 non polar amino acid residues
|
P-lease
M-ail I-n L-ove C-ards Tr-pping A Th-reesome Girly Gly |
|
|
What is the "window" in hydropathy plot?
|
sequence of 20 residues in which the calculations of free energy will determine whether they are imbedded o or not.
The calculations are plotted against the first amino acid at the start of the window and anything over +20 kcal/mol indicates a likely membrane spanning window. |
|
|
what is glycophorin?
|
an erythrocyte membrane protein that has a predicted membrane spanning region from a hydropathy plot which has been proven experimentally.
|
|
|
Are hydrophathy plots always correct?
|
No, even some soluable proteins contain large non-polar regions and some membrane spanning protein segments go undetected by hydropathy plots, like porin.
|
|
|
Describe the FRAP process of determining lateral diffusion
|
--cell surface fluoresces with labled surface
--fluorescent area is bleached and destroyed --fluorescence intensity recovers as diffusion occurs. --the rate of recover depends on the diffusion coefficient |
|
|
what is the diffusion coefficient of phospholipids?
|
1 Mm2s-1
diffuses ~2Mm / s (this ~100x that of water--more like olive oil) |
|
|
what is the diffusion coefficient of proteins?
what is a fast protein? What is a slow protein? |
a range
fast protein is photoreceptor protein rhodopsin 0.4 Mm2/s slow protein is peripheral glycoprotein fibronectin <10-4 Mm2/s |
|
|
Why is fibronectin a slow diffuser?
|
anchored to actin filaments through transmembrane protein integrin (stuck in the extracellular matrix and cytoskeleton)
|
|
|
what is the fluid mosaic model?
|
membranes are 2-D solutions of oriented lipids and globular proteins
lipid bilayer is a solvent for integral proteins and a permeability barrier membrane proteins are free to diffuse laterally in lipid matrix. |
|
|
what is transverse diffusion
|
flip flop.
takes a long time 10 to 9th as long as a lipid laterally diffuses 50 A. |
|
|
Where are PE and PS predominantly located?
|
The aminophospholipids, phosphatidylethanolamine (PE) and in particular phosphatidylserine (PS) are preferentially located in the inner leaflet of the membrane bilayer
|
|
|
Where are sphingomyelin and PC predominantly located?
|
the other major components sphingomyelin (SM) and phosphatidylcholine (PC) are more abundant in the outer leaflet
|
|
|
what are flippases?
|
translocater which flip flops PE and PS from outer to inner
|
|
|
What are floppases?
|
move phospholipids from inner to outer
needs ATP ABC transporter family |
|
|
What are scramblases?
|
move any phospholipid across bilayer down its concentration gradient
does not need ATP |
|
|
What is membrane fluidity depend upon?
|
The composition of fatty acids and their properties.
rigid fatty acids are saturated and unbent with lots of good H bonding and thus higher melting points more fluid membranes have unsaturated fatty acids that are bent and thus less H bonds and thus lower melting points. |
|
|
How does fatty acid chain length influence fluidity of membranes?
|
each additional -CH2- adds -0.5 kcal/mol to free energy of interaction between 2 adjacent hydrocarbon chains.
longer chains interact more strongly |
|
|
How does E. coli alter its fatty acid chains when it is trying to grow in colder temps?
|
decreases the ratio of saturated to unsaturated so that there are less rigid (saturated) fatty acids which prevents the membrane from becoming too rigid in this colder environment
|
|
|
How do animals regulate membrane fluidity?
|
cholesterol
|
|
|
what structurally about cholesterol helps it regulate membrane fluidity?
|
bulky steroid nuclues with a hydroxl group at one end and a flexible hydrocarbon tail at the other.
hydroxyl group interacts with aqueous environment (head group) hydrocarbon tail is in non-polar core |
|
|
what is a lipid raft?
|
cholesterol forms complexes with certain phospholipids concentrated in certain areas of the membrane.
These guys serve to moderate membrane fluidity. Makes them less fluid but also less likely to experience phase change |
|
|
asymmetric membranes means what?
|
inside and outside have different components and enzymes and thus different activities.
Na+ and K+ ATPase pump Na+ and ATP inside cell K+ outside |
|
|
what is ouabain?
|
cardiotonic steroid that inhibits the Na+ and K+ pumps
ONLY works on outside of cell. |

