![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
14 Cards in this Set
- Front
- Back
|
What is MAPK? |
- Mitogen activated protein kinase - serine/threonine kinase |
|
|
What are Ras proteins and how do they work? |
- subfamily of small GTPases - activated by SOS (which is activated by Growth factor receptor) - hydrolyse Raf and then - MEK -> MAPK |
|
|
Describe the Ras signalling cycle: |
- when inactive Ras has a GDP molecule bound to it - It GEF turns GDP to GTP - Ras is then active - GAP hydrolyses GTP back into GDP = inactive |
|
|
How is Ras recruited? |
- With growth factor receptors - treatment with EGF induces high levels of activated Ras - can also be activated by other receptors - GPCR which can stimulate different GEFs |
|
|
Describe the three tiered MAP kinase cascade: |
- MAPKKK (e.g. Raf) and MAPKs are serine/threonine kinases - MAPKK (e.g. MEK) are dual specific - phosphorilate both threonine and tyrosine residues on MAPK |
|
|
Three tiered MAP kinase cascade (image) |
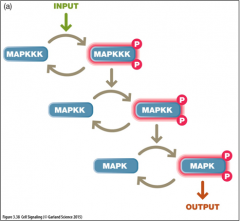
|
|
|
Different eukaryotic organisms vary in number of MAPK kinase: |
yes they do |
|
|
How are MAPK cascades organized? |
- By scaffold proteins - KSR, JIP, Ste5 and others |
|
|
Give example of a signalling molecule that starts a MAPK cascade |
- EGF family - HER2; - if deregulated - cancer |
|
|
How does the EGF - EGFR binding work? |
- whenEGF binds to EFGR monomer it alters the conformation of the extracellular domain, exposing the dimerization arm or "lip" - receptor dimerization causes activation of the tyrosine kinase domains which cross phosphorylate (transphosphorylation) - the phosphotyrosine residues provide docking sites for adaptor proteins such as Grb2 (which activates SOS, which hydrolyses Raf and so on) |
|
|
How is EGFR signalling switched off? |
- EGFR is mainly regulated through receptor degradation - phosphorylated EGFR recruits the ubiquitin ligase Cbl - the receptor becomes ubiquitinylated and internalised - targeted by the endocytotic pathway - the receptor is degraded by hydrolytic enzymes in the lysosome |
|
|
What mechanisms lead to abnormalities in EGFR signalling? |
- increased ligand production (cancer) - increased EGFR levels (gene amplification) - defective receptor downregulation - mutations - inappropriate dimarisation with other erbB family members |
|
|
What symptoms of cancer is EGFR associated with? |
- Metastasis - resistance to treatment |
|
|
What does RTK signalling in Drosophila and C. elegans do? |
- drosophila - eye formation - c. elegans - formation of the vulva |

